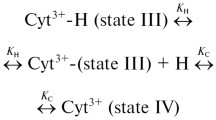Abstract
Resonance Raman, absorption and circular dichroism spectroscopic studies of the stable forms of horse heart ferricytochromec in thepH range 6–0.8 and at the lowest possible ionic strengths, in water, and at 30°C are reported. The neutralpH form, state III, changes to the acidicpH form, state I, through a three-step process: state III ↔ state IIIa ↔ state II ↔ state I, with pKa's of 3.6±0.3, 2.7±0.2, and 1.2±0.2, depending on the monitoring probe, respectively. State IIIa ferricytochromec is like state III (i.e., with the Met-80-sulfur-iron linkage and a closed heme crevice) but with a higher degree of folding and a slightly larger porphyrin core. State II ferricytochromec is an unfolded form with an open heme crevice and no Met-80-sulfur-iron linkage. The heme iron is high-spin and hexacoordinated with weak ligand-field groups, water, and nitrogen of the protonated/hydrogen-bonded imidazole of the His-18 residue at the axial positions. The state I form also lacks the Met-80-sulfur-iron linkage and has an open heme crevice like the state II form; however, it is less unfolded and has a high-spin pentacoordinated heme iron, with the nitrogen of the imidazole of His-18 as the axial ligate, which is out of the porphyrin plane by about 0.45 Å.
Similar content being viewed by others
References
Abe, M., Kitagawa, T., and Kyogoku, Y. (1978).J. Chem. Phys. 69, 4526–4534.
Asher, S. A. (1981).Meth. Enzymol. 76, 371–413.
Aviram, I. (1973).J. Biol. Chem. 248, 1894–1896.
Babul, J., and Stellwagen, E. (1972).Biochemistry 11, 1195–1200.
Boeri, E., Ehrenberg, A., Paul, K. G., and Theorell, H. (1953).Biochim. Biophys. Acta 12, 273–282.
Brautigan, D. L., Ferguson-Miller, S., and Margoliash, E. (1978).Meth. Enzymol. 53, 128–164.
Callahan, P. M., and Babcock, G. T. (1981).Biochemistry 20, 952–958.
Choi, S., Spiro, T. G., Langry, K. C., and Smith, K. M. (1982a).J. Am. Chem. Soc. 104, 4337–4344.
Choi, S., Spiro, T. G., Langry, K. C., Smith, K. M., Budd, D. L., and LaMar, G. N. (1982b).J. Am. Chem. Soc. 104, 4345–4351.
Cohen, J. S., and Hayes, M. B. (1974).J. Biol. Chem. 249, 5472–5477.
Dickerson, R. E., and Timkovich, R. (1975). InThe Enzymes (Boyer, P. D., ed.), Academic Press, New York, Vol. XIA, pp. 397–547.
Dayson, H. J., and Beattie, J. K. (1982).J. Biol. Chem. 257, 2267–2273.
Felton, R. H., Romans, A. Y., Yu, N.-T., and Schonbaum, G. R. (1976).Biochim. Biophys. Acta 434, 82.
Felton, R. H., and Yu, N.-T. (1978). InThe Porphyrins (Dolphin, D., ed.), Academic Press, New York, Vol. III, pp. 347–393.
Gupta, R. K., and Koenig, S. H. (1971).Biochem. Biophys. Res. Commun. 45, 1134–1143.
Harbury, H. A., and Loach, P. A. (1960).J. Biol. Chem. 235, 3640–3645, 3646–3653.
Harbury, H. A., and Marks, R. H. L. (1973). InInorganic Biochemistry (Eichorn, G. L., ed.), Elsevier, Amsterdam, pp. 902–956.
Hsu, M.-C., and Woody, R. W. (1971).J. Am. Chem. Soc. 93, 3515–3525.
Kastner, M. E., Scheidt, W. R., Mashiko, T., and Reed, C. A. (1978).J. Am. Chem. Soc. 100, 666–667.
Koenig, D. F. (1965).Acta Crystallogr. 18, 663–673.
Landrum, J. T., Hatano, K., Scheidt, W. R., and Reed, C. A. (1980).J. Am. Chem. Soc. 102, 6729–6737.
Lanir, A., Yu, N.-Y., and Felton, R. H. (1979).Biochemistry 18, 1656–1660.
Mashiko, T., Kastner, M. E., Spartalian, K., Scheidt, W. R., and Reed, C. A. (1978).J. Am. Chem. Soc. 100, 6354–6362.
Myer, Y. P. (1968).Biochemistry 7, 765–776.
Myer, Y. P. (1970).Res. Commun. Chem. Pathol. Pharmacol. 1, 607–616.
Myer, Y. P. (1972).Biochemistry 11, 4195–4203.
Myer, Y. P. (1985a). InOptical Properties and Structures of Tetrapyrroles (Blauer, G., and Sund, H., eds.), Walter de Gruyter, Berlin, pp. 203–226.
Myer, Y. P. (1985b). InCurrent Topics in Bioenergetics (Lee, C. P., ed.), Academic Press, New York, Vol. 14, pp. 149–188.
Myer, Y. P., and Harbury, H. A. (1973).Ann. N.Y. Acad. Sci. 206, 685–700.
Myer, Y. P., MacDonald, L. H., Verma, B. C., and Pande, A. J. (1980).Biochemistry 19, 199–207.
Myer, Y. P., and Pande, A. J. (1978). InThe Porphyrins (Dolphin D., ed.), Academic Press, New York, Vol. 3, pp. 271–322.
Myer, Y. P., Srivastava, R. B., Kumar, S., and Raghavendra, K. (1983).J. Protein Chem. 2, 13–42.
Peisach, J., Blumberg, W. E., Ogawa, S., Rachmilewitz, E. A., and Oltzik, R. (1971).J. Biol. Chem. 246, 3342–3355.
Robinson, J. B., Jr., Strottmann, J. M., and Stellwagen, E. (1983).J. Biol. Chem. 258, 6772–6776.
Scheidt, W. R. (1977).Accounts Chem. Res. 10, 339–345.
Scheidt, W. R., and Gouterman, M. (1983). InIron Porphyrins (Lever, A. B. P., and Grey, H. B., eds.), Addison-Wesley, London, Part I, pp. 89–132.
Schejter, A., Aviram, I., Margalit, R., and Goldkorn, T. (1975).Ann. N.Y. Acad. Sci. 244, 51–59.
Shechter, E., and Saludjian, P. (1967).Biopolymers 5, 788–790.
Smith, W. D., and Williams, R. J. P. (1970). InStructure and Bonding (Hemmerich, P., Jorgensen, C. K., Nyholm, R. S., and Williams, R. J. P., eds.), Springer-Verlag, New York, Vol. 7, pp. 1–45.
Spaulding, L. D., Change, C. C., Yu, N.-T., and Felton, R. H. (1975).J. Am. Chem. Soc. 97, 2517–2525.
Spiro, T. G. (1983). InIron Porphyrins (Lever, A. B. P., and Grey, H. B., eds.), Addison-Wesley, Reading, Massachusetts, Part II, pp. 89–160.
Spiro, T. G., Stong, J. D., and Stein, P. (1979).J. Am. Chem. Soc. 101, 2648–2685.
Strekas, T. C., and Spiro, T. G. (1974).Biochim. Biophys. Acta 351, 237–245.
Sreenathan, B. R., and Taylor, C. R. S. (1971).Biochem. Biophys. Res. Commun. 42, 1122–1126.
Takano, T., Trus, B. L., Mandel, N., Mandel, G., Kalai, O. B., Swanson, R., and Dickerson, R. E. (1977).J. Biol. Chem. 252, 776–785.
Theorell, H. (1941).J. Am. Chem. Soc. 63, 1820–1827.
Theorell, H., and Akenson, A. (1941).J. Am. Chem. Soc. 63, 1804–1820.
Weber, P. C. (1982).Biochemistry 21, 5116–5119.
Weber, P. C., Howard, A., Xuong, Ng. H., and Salemme, F. R. (1981).J. Mol. Biol. 153, 399–424.
Wooten, J. B., Cohen, J. S., Vig, I., and Schejter, A. (1981).Biochim. Biophys. Acta 439, 232–239.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Myer, Y.P., Saturno, A.F. Horse heart ferricytochromec: Conformation and heme configuration of low ionic strength acidic forms. J Protein Chem 9, 379–387 (1990). https://doi.org/10.1007/BF01024613
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01024613