Abstract
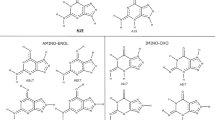
The acidity constants of the two-fold protonated acyclic 9-[2-(phosphonomethoxy)ethyl]-8-azaadenine, H2(9,8aPMEA)±, and its 8-isomer, 8-[2-(phosphonomethoxy)ethyl]-8-azaadenine, H2(8,8aPMEA)±, both abbreviated as H2(PA)±, as well as the stability constants of their M(H;PA)+ and M(PA) complexes with the metal ions M2+=Mg2+, Ca2+, Sr2+, Ba2+, Mn2+, Co2+, Ni2+, Cu2+, Zn2+ or Cd2+, have been determined by potentiometric pH titrations in aqueous solution at I=0.1 M (NaNO3) and 25 °C. Application of previously determined straight-line plots of log \( K_{{\text{M(R-PO}}_{\text{3}} {\text{)}}}^{\text{M}} \) versus \( {\text{p}}K_{{\text{H(R-PO}}_{\text{3}} {\text{)}}}^{\text{H}} \) for simple phosph(on)ate ligands, \( {\text{R-PO}}_3^{2-} \), where R represents a residue without an affinity for metal ions, proves that for all M(PA) complexes a larger stability is observed than is expected for a sole phosphonate coordination of the metal ion. This increased stability is attributed to the formation of five-membered chelates involving the ether oxygen present in the aliphatic residue (\( {\text{-CH}}_{\text{2}} {\text{-O-CH}}_{\text{2}} {\text{-PO}}_{\text{3}}^{{\text{2}}-} \)) of the ligands. The formation degrees of these chelates were calculated; they vary between about 13% for Ca(8,8aPMEA) and 71% for Cu(8,8aPMEA). The adenine residue has no influence on complex stability except in the Cu(9,8aPMEA) and Zn(9,8aPMEA) systems, where an additional stability increase attributable to the adenine residue is observed and equilibria between four different isomers exist. This means (1) an open isomer with a sole phosphonate coordination, M(PA)op, where PA2−=9,8aPMEA2−, (2) an isomer with a five-membered chelate involving the ether oxygen, M(PA)cl/O, (3) an isomer which contains five- and seven-membered chelates formed by coordination of the phosphonate group, the ether oxygen and the N3 site of the adenine residue, M(PA)cl/O/N3, and finally (4) a macrochelated isomer involving N7, M(PA)cl/N7. For Cu(9,8aPMEA) the formation degrees are 15, 30, 48 and 7% for Cu(PA)op, Cu(PA)cl/O, Cu(PA)cl/O/N3 and Cu(PA)cl/N7, respectively; this proves that the macrochelate involving N7 is a minority species. The situation for the Cu(PMEA) system, where PMEA2− represents the parent compound, i.e. the dianion of 9-[2-(phosphonomethoxy)ethyl]adenine, is quite similar. The relationship between the antiviral activity of acyclic nucleoside phosphonates and the structures of the various complexes is discussed and an explanation is offered why 9,8aPMEA is biologically active but 8,8aPMEA is not.


Similar content being viewed by others
Notes
Species written without a charge either do not carry one or represent the species in general (i.e. independent of their protonation degree); which of the two possibilities applies is always clear from the context. In formulas like M(H;PA)+, the H+ and PA2− are separated by a semicolon to facilitate reading, yet they appear within the same parenthesis to indicate that the proton is at the ligand without defining its location
Information regarding US FDA: (2002) Chem Rundschau (CH-4501 Solothurn, Switzerland) no. 19 (Oct 8), p 68
Information regarding EMEA as downloaded from the World Wide Web in December 2003: http://www.emea.eu.int/humandocs/PDFs/EPAR/hepsera/610202en1.pdf
Abbreviations
- (d)ATP4−:
-
(2′-deoxy)adenosine 5′-triphosphate
- PMEA:
-
9-[2-(phosphonomethoxy)ethyl]adenine
- 8,8aPMEA:
-
8-[2-(phosphonomethoxy)ethyl]-8-azaadenine
- 9,8aPMEA:
-
9-[2-(phosphonomethoxy)ethyl]-8-azaadenine
- I :
-
ionic strength
- K a :
-
acidity constant
- M2+:
-
divalent metal ion
References
Tamm I, Folkers K, Shunk CH (1956) J Bacteriol 72:59–64
Martin JC (ed) (1989) Nucleotide analogs as antiviral agents. (ACS symposium series 401) American Chemical Society, Washington, DC, pp 1–190
Sigel RKO, Song B, Sigel H (1997) J Am Chem Soc 119:744–755
Holý A (2003) Curr Pharm Des 9:2567–2592
Holý A, Günter J, Dvořáková H, Masojídková M, Andrei G, Snoeck R, Balzarini J, De Clercq E (1999) J Med Chem 42:2064–2086
Holý A, Votruba I, Masojídková M, Andrei G, Snoeck R, Naesens L, De Clercq E, Balzarini J, (2002) J Med Chem 45:1918–1929
De Clercq E (1998) Collect Czech Chem Commun 63:449–479
De Clercq E (1998) Collect Czech Chem Commun 63:480–506
Keith KA, Hitchcock MJM, Lee WA, Holý A, Kern ER (2003) Antimicrob Agents Chemother 47:2193–2198
De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC (1986) Nature 323:464–467
Tribolet R, Sigel H (1987) Eur J Biochem 163:353–363
Aoki K (1996) Met Ions Biol Syst 32:91–134
Blindauer CA, Holý A, Dvořáková H, Sigel H (1997) J Chem Soc Perkin Trans 2 2353–2363
Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J (1994) Science 264:1891–1903
Pelletier H, Sawaya MR, Wolfle W, Wilson SH, Kraut J (1996) Biochemistry 35:12762–12777
Brautigam CA, Steitz TA (1998) Curr Opinion Struct Biol 8:54–63
Sigel H (1992) Inorg Chim Acta 198–200:1-11
Sigel H (1990) Coord Chem Rev 100:453–539
Sigel H, Song B, Blindauer CA, Kapinos LE, Gregáň F, Prónayová N (1999) Chem Commun 743–744
Holý A, De Clercq E, Votruba I (1989) ACS Symp Ser 401:51–71
Holý A, Votruba I, Merta A, Černý J, Veselý J, Vlach J, Šedivá K, Rosenberg I, Otmar M, Hřebabecký H, Trávníček M, Vonka V, Snoeck R, De Clercq E (1990) Antiviral Res 13:295–311
Sigel H (1999) Pure Appl Chem 71:1727–1740
Sigel H (2004) Chem Soc Rev 33:191–200
Villemin D, Thibault-Starzyk F (1993) Synth Commun 23:1053–1059
Holý A, Rosenberg I, Dvořáková H (1990) Collect Czech Chem Commun 55:809–818
Holý A, Dvořáková H, Jindřich J, Masojídková M, Buděšínský M, Balzarini J, Andrei G, De Clercq E (1996) J Med Chem 39:4073–4088
Roblin RO Jr, Lampen JO, English JP, Cole QP, Vanghan JR Jr (1945) J Am Chem Soc 67:290–294
Kidder GW, Dewey VC, Parks RE Jr, Woodside GL (1949) Science 109:511–514
Singh P, Hodgson DJ (1977) J Am Chem Soc 99:4807–4815
Sheldrick WS, Bell P (1986) Inorg Chim Acta 123:181–187
Sheldrick WS, Bell P (1989) Inorg Chim Acta 160:265–271
Franchetti P, Abu Sheikha G, Cappellacci L, Grifantini M, De Montis A, Piras G, Loi AG, La Colla P (1995) J Med Chem 38:4007–4013
Dvořáková H, Holý A, Masojídková M, Votruba I, Balzarini J, Snoeck R, De Clercq E (1993) Collect Czech Chem Commun 58:253–255
Sigel H (1995) Coord Chem Rev 144:287–319
Blindauer CA, Emwas AH, Holý A, Dvořáková H, Sletten E, Sigel H (1997) Chem Eur J 3:1526–1536
Blindauer CA, Holý A, Dvořáková H, Sigel H (1998) J Biol Inorg Chem 3:423–433
Sigel H, Massoud SS, Tribolet R (1988) J Am Chem Soc 110:6857–6865
Sigel H (1993) Chem Soc Rev 22:255–267
Sigel H (2004) Pure Appl Chem 76:375–388
Bianchi EM, Sajadi SAA, Song B, Sigel H (2003) Chem Eur J 9:881–892
Gómez-Coca RB, Holý A, Vilaplana RA, González-Vílchez F, Sigel H (2004) Bioinorg Chem Applicat 2:(in press)
Gómez-Coca RB, Kapinos LE, Holý A, Vilaplana RA, González-Vílchez F, Sigel H (2000) Metal Based Drugs 7:313–324
Sigel H, Zuberbühler AD, Yamauchi O (1991) Anal Chim Acta 255:63–72
Irving HM, Miles MG, Pettit LD (1967) Anal Chim Acta 38:475–488
Blindauer CA, Sjåstad TI, Holý A, Sletten E, Sigel H (1999) J Chem Soc Dalton Trans 3661–3671
Gómez-Coca RB, Kapinos LE, Holý A, Vilaplana RA, González-Vílchez F, Sigel H (2000) J Chem Soc Dalton Trans 2077–2084
Martin RB, Sigel H (1988) Comments Inorg Chem 6:285–314
Kapinos LE, Song B, Sigel H (1999) Chem Eur J 5:1794–1802
Kapinos LE, Song B, Sigel H (1998) Inorg Chim Acta 280:50–56
Kapinos LE, Sigel H (2002) Inorg Chim Acta 337:131–142
Sigel H, Chen D, Corfù NA, Gregáň F, Holý A, Strašák M (1992) Helv Chim Acta 75:2634–2656
Yamauchi O, Odani A, Masuda H, Sigel H (1996) Met Ions Biol Syst 32:207–270
Scheller KH, Hofstetter F, Mitchell PR, Prijs B, Sigel H (1981) J Am Chem Soc 103:247–260
Kampf G, Kapinos LE, Griesser R, Lippert B, Sigel H (2002) J Chem Soc Perkin Trans 2 1320–1327
Sigel H (2004) Pure Appl Chem 76:(in press)
Sheldrick WS, Heeb G (1991) Inorg Chim Acta 190:241–248
Albert A (1969) J Chem Soc (C) 152–160
Blindauer CA, Holý A, Sigel H (1999) Collect Czech Chem Commun 64:613–632
Kapinos LE, Kampf G, Griesser R, Lippert B, Sigel H (1999) Chimia 53:348
Sigel H, Da Costa CP, Song B, Carloni P, Gregáň F (1999) J Am Chem Soc 121:6248–6257
Da Costa CP, Sigel H (1999) J Biol Inorg Chem 4:508–514
Sigel H, Massoud SS, Corfù NA (1994) J Am Chem Soc 116:2958–2971
Sigel H, McCormick DB (1970) Acc Chem Res 3:201–208
Saha A, Saha N, Ji L, Zhao J, Gregáň F, Sajadi SAA, Song B, Sigel H (1996) J Biol Inorg Chem 1:231–238
Sigel H, Song B (1996) Met Ions Biol Syst 32:135–205
Sajadi SAA, Song B, Gregáň F, Sigel H (1999) Inorg Chem 38:439–448
Irving HM, Williams RJP (1953) J Chem Soc: 3192–3210
Sigel H, Lippert B (1998) Pure Appl Chem 70:845–854
Griesser R, Kampf G, Kapinos LE, Komeda S, Lippert B, Reedijk J, Sigel H (2003) Inorg Chem 42:32–41
Sigel H, Corfù NA, Ji L-n, Martin RB (1992) Comments Inorg Chem 13:35–59
Martin RB (1996) Met Ions Biol Syst 32:61–89
Sigel H, Kapinos LE (2000) Coord Chem Rev 200–202:563–594
Massoud SS, Sigel H (1988) Inorg Chem 27:1447–1453
Franchetti P, Abu Sheikha G, Cappellacci L, Messini L, Grifantini M, Loi AG, De Montis A, Spiga MG, La Colla P (1994) Nucleosides Nucleotides 13:1707–1719
Merta A, Votruba I, Rosenberg I, Otmar M, Hřebabecký H, Bernaerts R, Holý A (1990) Antiviral Res 13:209–218
Robbins BL, Greenhaw J, Connelly MC, Fridland A (1995) Antimicrob Agents Chemother 39:2304–2308
Kramata P, Votruba I, Otová B, Holý A (1996) Mol Pharmacol 49:1005–1011
Birkuš G, Votruba I, Holý A, Otová B (1999) Biochem Pharmacol 58:487–492
Acknowledgements
The competent technical assistance of Mrs Rita Baumbusch and Mrs Astrid Sigel in the preparation of this manuscript as well as stimulating discussions with members of the COST D20 programme are gratefully acknowledged. This study was supported by the Swiss National Science Foundation (H.S.) and the Programme of Targeted Projects (S4055109) of the Academy of Sciences of the Czech Republic (A.H.) as well as within the COST D20 programme by the Swiss Federal Office for Education and Science (H.S.) and the Ministry of Education of the Czech Republic (D20.002; A.H.). This study also received support from the University of Basel and it is further part of a research project (no 4055905) of the Institute of Organic Chemistry and Biochemistry (IOCB) in Prague.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Coca, R.B., Kapinos, L.E., Holý, A. et al. Quantification of isomeric equilibria formed by metal ion complexes of 8-[2-(phosphonomethoxy)ethyl]-8-azaadenine (8,8aPMEA) and 9-[2-(phosphonomethoxy)ethyl]-8-azaadenine (9,8aPMEA). Derivatives of the antiviral nucleotide analogue 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA). J Biol Inorg Chem 9, 961–972 (2004). https://doi.org/10.1007/s00775-004-0591-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0591-7