Abstract
Introduction
Bone homeostasis depends on the regulation of β-catenin in osteoblasts. Glucocorticoids (GCs) are known to diminish β-catenin activity via Wnt pathway signaling, leading to osteoporosis. Conversely, activating β-catenin in osteoblasts through mitogen-activated protein kinase kinase kinase 2 (Mekk2) offers an innovative approach to combat GC-induced osteoporosis (GIOP). Fufang Zhenshu Tiaozhi (FTZ) capsules have shown effectiveness in treating GIOP, but the mechanisms behind this are still unclear.
Materials and methods
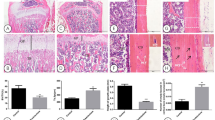
In this study, Mekk2 knockout mice (Mekk2−/−) was generated by CRISPR/Cas9. These mice were then subjected to Alcian Blue-Alizarin Red staining and immunofluorescence to assess their bone and cartilage development. To establish models of GIOP, both Mekk2−/− and wild-type (WT) mice were treated with dexamethasone (DXMS) and subsequently given FTZ capsules. We analyzed the resulting phenotypic changes in these mice using Micro-CT scans and histomorphological studies. Primary osteoblasts, isolated from both Mekk2−/− and WT mice, underwent qRT-PCR to measure key osteogenesis markers, including Runx2, Sp7, Bgalp, Col1a1 and Alp. Cells were then exposed to treatments with either FTZ or Wnt3a and the phosphorylation levels of β-catenin and Mekk2, along with the protein expression of Runx2, were evaluated using Western blotting and immunoprecipitation. Additionally, C3H10T1/2 cells transfected with TOPflash-luciferase and Renilla luciferase reporters were treated with FTZ and Wnt3a to measure β-catenin activity.
Results
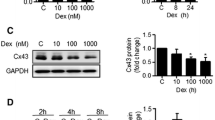
In our study, administering FTZ in vivo effectively prevented bone loss typically induced by GCs. However, it's important to note that this protective effect was substantially reduced in mice lacking Mekk2. Additionally, FTZ showed a significant ability to enhance osteogenic differentiation in primary osteoblasts, doing so by altering the expression of Mekk2. Intriguingly, the impact of FTZ on Mekk2 appears to function through a pathway separate from the traditional Wnt signaling route. Furthermore, our findings indicate that FTZ also promotes the deubiquitination of β-catenin, contributing further to its positive effects on bone health.
Conclusions
This study suggests that FTZ plays a significant role in protecting bone mass in cases of GIOP. The mechanism through which FTZ confers this benefit involves the activation of Mekk2/β-catenin signaling pathways, which represents a promising alternative strategy to counteract the deleterious effects of GIOP by augmenting osteoblastogenesis.







Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GC:
-
Glucocorticoid
- FTZ:
-
Fufang Zhenshu Tiaozhi
- GIOP:
-
Glucocorticoid-induced osteoporosis
- Mekk2:
-
Mitogen-activated protein kinase kinase kinase 2
- DXMS:
-
Dexamethasone
- WT:
-
Wild type
- ALP:
-
Alkaline phosphatase
References
Buckley L, Humphrey MB (2018) Glucocorticoid-induced osteoporosis. N Engl J Med 379:2547–2556. https://doi.org/10.1056/NEJMcp1800214
Chotiyarnwong P, McCloskey EV (2020) Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol 16:437–447. https://doi.org/10.1038/s41574-020-0341-0
Gregson CL, Armstrong DJ, Bowden J et al (2022) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. https://doi.org/10.1007/s11657-022-01061-5
Adami G, Saag KG (2019) Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol 31:388–393. https://doi.org/10.1097/BOR.0000000000000608
Watts NB, GLOW investigators, (2014) Insights from the global longitudinal study of osteoporosis in women (GLOW). Nat Rev Endocrinol 10:412–422. https://doi.org/10.1038/nrendo.2014.55
Silverman S, Curtis J, Saag K et al (2015) International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int 26:419–420. https://doi.org/10.1007/s00198-014-2883-2
Meszaros K, Patocs A (2020) Glucocorticoids Influencing Wnt/β-Catenin Pathway; Multiple Sites. Heterogeneous Eff Mol 25:1489. https://doi.org/10.3390/molecules25071489
Komori T (2016) Glucocorticoid signaling and bone biology. Horm Metab Res 48:755–763. https://doi.org/10.1055/s-0042-110571
Greenblatt MB, Shin DY, Oh H et al (2016) MEKK2 mediates an alternative β-catenin pathway that promotes bone formation. Proc Natl Acad Sci U S A 113:E1226-1235. https://doi.org/10.1073/pnas.1600813113
Yamashita M, Ying S-X, Zhang G-M et al (2005) Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 121:101–113. https://doi.org/10.1016/j.cell.2005.01.035
Lawson LY, Brodt MD, Migotsky N et al (2022) Osteoblast-specific Wnt secretion is required for skeletal homeostasis and loading-induced bone formation in adult mice. J Bone Miner Res 37:108–120. https://doi.org/10.1002/jbmr.4445
Karner CM, Long F (2017) Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci 74:1649–1657. https://doi.org/10.1007/s00018-016-2425-5
Chen X, Wang J, Tang L et al (2022) The therapeutic effect of Fufang Zhenshu Tiaozhi (FTZ) on osteoclastogenesis and ovariectomized-induced bone loss: evidence from network pharmacology, molecular docking and experimental validation. Aging (Albany NY) 14:5727–5748. https://doi.org/10.18632/aging.204172
Luo D, Li J, Chen K et al (2018) Untargeted metabolomics reveals the protective effect of Fufang Zhenshu Tiaozhi (FTZ) on aging-induced osteoporosis in mice. Front Pharmacol 9:1483. https://doi.org/10.3389/fphar.2018.01483
Huang X, Zhan H, Yang J et al (2021) Long-term effect of Zhenzhu Tiaozhi Capsule (FTZ) on hyperlipidemia: 2-year results from a retrospective study using electronic medical records. Evid Based Complement Alternat Med 2021:6264414. https://doi.org/10.1155/2021/6264414
Luo D, Chen K, Li J et al (2020) Gut microbiota combined with metabolomics reveals the metabolic profile of the normal aging process and the anti-aging effect of FuFang Zhenshu TiaoZhi(FTZ) in mice. Biomed Pharmacother 121:109550. https://doi.org/10.1016/j.biopha.2019.109550
Diao H, Cheng J, Huang X et al (2022) The Chinese medicine Fufang Zhenzhu Tiaozhi capsule protects against atherosclerosis by suppressing EndMT via modulating Akt1/β-catenin signaling pathway. J Ethnopharmacol 293:115261. https://doi.org/10.1016/j.jep.2022.115261
Wang H, Tan H, Zhan W et al (2021) Molecular mechanism of Fufang Zhenzhu Tiaozhi capsule in the treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease based on network pharmacology and validation in minipigs. J Ethnopharmacol 274:114056. https://doi.org/10.1016/j.jep.2021.114056
Guo J, Bei W, Hu Y et al (2011) A new TCM formula FTZ lowers serum cholesterol by regulating HMG-CoA reductase and CYP7A1 in hyperlipidemic rats. J Ethnopharmacol 135:299–307. https://doi.org/10.1016/j.jep.2011.03.012
Xu Y, Tang J, Guo Q et al (2021) Traditional Chinese medicine formula FTZ protects against polycystic ovary syndrome through modulating adiponectin-mediated fat-ovary crosstalk in mice. J Ethnopharmacol 268:113587. https://doi.org/10.1016/j.jep.2020.113587
Komori T (2015) Animal models for osteoporosis. Eur J Pharmacol 759:287–294. https://doi.org/10.1016/j.ejphar.2015.03.028
Lane NE (2019) Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Curr Osteoporos Rep 17:1–7. https://doi.org/10.1007/s11914-019-00498-x
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328. https://doi.org/10.1007/s00198-007-0394-0
Kenkre JS, Bassett J (2018) The bone remodelling cycle. Ann Clin Biochem 55:308–327. https://doi.org/10.1177/0004563218759371
An J, Yang H, Zhang Q et al (2016) Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci 147:46–58. https://doi.org/10.1016/j.lfs.2016.01.024
Wang T, Liu Q, Tjhioe W et al (2017) Therapeutic potential and outlook of alternative medicine for osteoporosis. Curr Drug Targets 18:1051–1068. https://doi.org/10.2174/1389450118666170321105425
Jiang Y, Lu Y, Jiang X et al (2020) Glucocorticoids induce osteoporosis mediated by glucocorticoid receptor-dependent and -independent pathways. Biomed Pharmacother 125:109979. https://doi.org/10.1016/j.biopha.2020.109979
Messina OD, Vidal M, Torres JAM et al (2022) Evidence based Latin American guidelines of clinical practice on prevention, diagnosis, management and treatment of glucocorticoid induced osteoporosis. A 2022 update : This manuscript has been produced under the auspices of the committee of national societies (CNS) and the committee of scientific advisors (CSA) of the international osteoporosis foundation (IOF). Aging Clin Exp Res 34:2591–2602. https://doi.org/10.1007/s40520-022-02261-2
Latifyan S, Genot MT, Klastersky J (2016) Bisphosphonate-related osteonecrosis of the jaw: a review of the potential efficacy of low-level laser therapy. Support Care Cancer 24:3687–3693. https://doi.org/10.1007/s00520-016-3139-9
Janovská Z (2012) Bisphosphonate-related osteonecrosis of the jaws. A severe side effect of bisphosphonate therapy. Acta Medica (Hradec Kralove) 55:111–115. https://doi.org/10.14712/18059694.2015.47
Jiang L, Dong J, Wei J, Liu L (2022) Comparison of denosumab and oral bisphosphonates for the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. BMC Musculoskelet Disord 23:1027. https://doi.org/10.1186/s12891-022-05997-0
Otto S, Pautke C, Van den Wyngaert T et al (2018) Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev 69:177–187. https://doi.org/10.1016/j.ctrv.2018.06.007
Butz H, Mészáros K, Likó I, Patocs A (2021) Wnt-Signaling Regulated by Glucocorticoid-Induced miRNAs. Int J Mol Sci 22:11778. https://doi.org/10.3390/ijms222111778
Rossini M, Gatti D, Adami S (2013) Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int 93:121–132. https://doi.org/10.1007/s00223-013-9749-z
Saidak Z, Marie PJ (2012) Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther 136:216–226. https://doi.org/10.1016/j.pharmthera.2012.07.009
Wan L, Zou W, Gao D et al (2011) Cdh1 regulates osteoblast function through an APC/C-independent modulation of Smurf1. Mol Cell 44:721–733. https://doi.org/10.1016/j.molcel.2011.09.024
Acknowledgements
Not applicable.
Funding
This study was supported in part by the National Natural Science Foundation of China (No. 81704098, 82305265), Science and Technology Planning Project of Yunfu (No. 2020A090402), Guangdong Science and Technology Innovation Strategy Special Funding "Great Special Project + Task List" (No. 2020A090402), and China Postdoctoral Science Foundation (grant number: 2023M730809). This sponsor hasn’t participated in the design of the study or collection, analysis, or interpretation of data or in writing manuscript.
Author information
Authors and Affiliations
Contributions
Ping Sun and Qunwei Dong conceived and supervised the study; Guoju Hong designed experiments; Guoju Hong, Jiangyan Wang and Dongdong Ge performed experiments; Lin Tang provided new tools and reagents; Tianyu Zhou and Youhong Xie analysed data; Guoju Hong wrote the manuscript; Ping Sun made manuscript revisions.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The animal protocol was approved by the animal ethics broad of Guangzhou University of Chinese Medicine where the animal study was performed.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hong, G., Tang, L., Zhou, T. et al. Fufang Zhenshu Tiaozhi capsule enhances bone formation and safeguards against glucocorticoid-induced osteoporosis through innovative Mekk2-mediated β-catenin deubiquitination. J Bone Miner Metab (2024). https://doi.org/10.1007/s00774-024-01516-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00774-024-01516-4