Abstract
Introduction
Nutritional prevention of osteoporosis management is an important issue for children with severe disabilities. Due to the coronavirus disease 2019 (COVID-19) pandemic that started in 2020, children admitted to institutions had fewer opportunities for ultraviolet (UV) exposure owing to restrictions on attending school and going out. Hence, the vitamin D (VD) status of these children has been a cause of concern. This study aimed to assess the correlation between VD intake and VD status among children with severe disabilities who had limited UV exposure.
Materials and methods
This research included patients admitted to Iwate Prefectural Rehabilitation and Nursery Center for Disabled Children. Serum 25-hydroxyvitamin D [25(OH)D] levels were assessed during school/outing restriction periods and after restriction removal and the introduction of sunbathing periods. The trends in 25(OH)D levels and oral VD intake before the two measurements were analyzed.
Results
Although 17 of 32 patients had VD intake above the recommended level of Dietary Reference Intakes for Japanese during the first measurement, 31 patients had VD deficiency. The 25(OH)D levels of 13 patients without UV exposure before the first evaluation and those with UV exposure before the second evaluation were 2.03 times higher, despite of constant VD intakes. In contrast, there were no remarkable changes in both VD intakes and 25(OH)D levels in five patients without UV exposure before both assessments.
Conclusion
Japanese children with severe disabilities who consume the recommended oral VD intake but who have limited UV exposure can still present VD deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with severe disabilities have low bone mineral density due to the lack of antigravity loading, decreased or limited sun exposure, and use of antiepileptic drugs. Hence, vitamin D (VD) is an important factor for preventing osteoporosis and bone fractures for these children [1]. Moreover, VD has other functions such as enhancing immunity and inhibiting the development of cancer and diabetes making it essential in the healthcare of children with severe disabilities [2, 3]. Dietary intake and synthesis in the skin via ultraviolet (UV) exposure are two sources of VD. Serum 25 hydroxyvitamin D [25(OH)D] level is a useful indicator of VD status.
UV exposure plays a major role in VD status [4]. However, data about changes in the VD status of children with severe disabilities based on UV exposure are limited. Due to the outbreak of coronavirus disease 2019 (COVID-19), inpatients at Iwate Prefectural Rehabilitation and Nursery Center for Disabled Children were restricted from attending outings and school since April 2020. This then reduced the opportunities for UV exposure. In July, we stopped restricting patients from attending school, and some started sunbathing in the courtyard. Subsequently, to evaluate the VD status of patients, two blood tests were conducted before and after the removal of school attendance restrictions and the introduction of sunbathing. Moreover, the VD intake of patients was calculated before the assessments. The current study aimed to validate the association between vitamin D intake and 25(OH)D levels among children with severe disabilities who had limited UV exposure.
Materials and methods
Participants (Table 1 )
In total, 36 patients had been admitted to Iwate Prefectural Rehabilitation and Nursery Center for Disabled Children since November 1, 2020. Of these, 32 patients were included in the current study, and these patients underwent blood tests in May to June (phase 1) and October to November (phase 2). The blood test of the remaining four patients was enforced only in one of the two phases. Our center is located at 39°36′N. The windows of the rooms are not opened, and there is no opportunity for UV exposure except when going out or sunbathing in the courtyard. In total, 16 (50%) patients were boys and 16 (50%) were girls. The age ranged from 1 year and 10 months to 18 years and 6 months (median: 9 years and 3 months). The nutritional methods were tube feeding (n = 15), oral intake (n = 16) and combined feeding (n = 1). The underlying diseases were hypoxic-ischemic encephalopathy (HIE) in 12 patients; muscular dystrophy, three patients; chromosomal abnormality, three patients; sequelae of encephalopathy, two patients; hydrocephalus, two patients; and schizencephaly, subdural hematoma, multiple malformations, congenital cytomegalovirus (CMV) infection, Angelman syndrome, sequelae of bacterial meningitis, sequelae of brain tumor, spinal muscular atrophy, and brain malformation, one patient each. The Gross Motor Function Classification System (GMFCS) level was III in two patients, IV, 7 patients and V, 23 patients. Five patients were on ventilators all day, and three patients used ventilators at night. In total, 17 patients were taking blood VD-lowering medications, such as valproic acid (n = 11) and phenobarbital (n = 8) [5]. That is, three patients were taking alfacalcidol (Alfarol®), and one was treated with comprehensive vitamin preparation (Panvitan®), including ergocalciferol (VD2).
Examinations
This is a before and after study. The serum 25(OH)D levels were assessed for the first time (phase 1) from May to June 2020 and for the second time (phase 2) from October to November 2020. The assessments were performed by LSI Medience Corporation using the chemiluminescent enzyme immunoassay method. We calculated the VD intake for 30 consecutive days before phases 1 and 2. The VD intake per day was evaluated based on the VD content of enteral nutrition according to the package insert, and the content of blended diet based on the nutrition department of our institution and the average daily dose for 30 days. Oral intake was calculated according to the VD content of the dietary prescriptions of the nutrition department of our institution and the dietary intake for 30 days. For patients taking oral comprehensive vitamins, the oral medication and meal intakes were calculated. The recommended daily allowances (RDAs) for each age and sex were calculated based on the Dietary Reference Intakes for Japanese (2020) by the Study Group of Ministry of Health, Labour and Welfare [6]. We used the actual intake-to-RDA ratio according to each age and sex as an indicator of the intake of each patient. The RDAs based on each age and sex were based on the age of patients since November 1, 2020. VD deficiency was defined as a serum 25(OH)D level of < 20 ng/mL and VD insufficiency as serum 25(OH)D level between 20 and 30 ng/mL based on the assessment criteria for vitamin D deficiency/insufficiency by the Japanese Endocrine Society and the Japanese Society for Bone and Mineral Research [7].
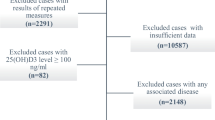
The half-life of 25(OH)D is approximately 15 days [8]. Hence, we investigated whether the patients attended school, went out, or sunbathed within 15 days before phases 1 and 2 based on the medical records and data on leisure activities. Patients who attended school, went out, or sunbathed at least once within 15 days before phase 1 were included in group A and those who did not were included in group B or C. Patients in group B or C who attended school, went out, or sunbathed at least once within 15 days before phase 2 were included in group B and those who did not were included in group C (Fig. 1). In groups A + B, A, B and C, changes in the VD intake-to-RDA ratio, which is an indicator of intake, and serum 25(OH)D levels between phase 1 and 2 were examined. We calculated the VD intake-to-RDA ratio before phases 1 and 2 in groups A, B, and C, respectively.
The Wilcoxon’s signed-rank sum test was used for statistical analysis with statistical significance set at P < 0.05. Microsoft Office Excel 2019 was used for statistical analysis.
Results
Participants were allocated to 1 of 3 groups (Fig. 1). There were 14 patients in group A, 13 in group B and 5 in group C (Table 1). All patients in group C did not have opportunities to attend school, go on outings, or sunbathe due to the need of a ventilator throughout the day and their unstable general condition. In total, 20 school-aged patients in groups A and B resumed attending school in July 2020. Moreover, 7 of the 12 children who were in good general condition and did not need to use ventilator throughout the day were actively sunbathing inside the institution. Oral intake was limited in some patients due to difficulties in oral administration because of neuromuscular diseases. Additionally, some patients were restricted in the amount of diet to treat obesity. Only patient no. 29 had been diagnosed with urinary tract stones.
Phase 1
The serum 25(OH)D levels of the patients ranged from 4.3 to 20.2 ng/mL, with a median value of 9.2 ng/mL. In total, 31 patients had VD deficiency (< 20 ng/mL), and one presented with VD insufficiency (20–30 ng/mL) (Table 1 and Fig. 2). The average VD intakes within 30 consecutive days before phase 1 were higher than the RDA according to age and sex in 17 patients and lower in 15 patients. The VD intake-to-RDA ratio ranged from 0.53 to 2.43 (median: 1.01) (Table 1 and Fig. 2).
Phase 2
The serum 25(OH)D levels ranged from 6.5 to 30.1 (median: 18.3) ng/mL. In total, 21 patients had VD deficiency (< 20 ng/mL), and 10 patients presented with VD insufficiency (20–30 ng/mL). In groups A and B, all patients had elevated serum 25(OH)D levels (Table 1 and Fig. 2). The average VD intakes within 30 consecutive days before phase 2 were higher than the RDA for each age and sex in 21 patients and lower in 11 patients. The VD intake-to-RDA ratio ranged from 0.57 to 2.29 (median: 1.22) (Table 1 and Fig. 2).
Statistical analysis
In groups A and B (n = 27), the VD intake-to-RDA ratio did not increase significantly (P = 0.073), whereas the serum 25(OH)D level increased significantly (P < 0.001). In group B (n = 13), the VD intake-to-RDA ratio did not increase significantly (P = 0.23). Meanwhile, the serum 25(OH)D level increased significantly (P = 0.0015) from phase 1 to 2. The serum 25(OH)D level increased by 2.03-fold from phase 1 to 2 in group B. In group C (n = 5), there was no significant increase in the VD intake-to-RDA ratio (P = 0.12) and the serum 25(OH)D level (P = 0.38) did not change significantly from phase 1 to 2.
Discussion
Due to the COVID-19 outbreak, inpatients at the Iwate Prefectural Rehabilitation and Nursery Center for Disabled Children had been restricted from attending outings and school since April 2020, reducing opportunities for UV exposure. In July 2020, these restrictions were eased and some patients were actively taken out to sunbathe in the courtyard. This study evaluated VD status before and after this change. Following extremely low UV exposure, almost all the patients were VD deficient, even though their VD intake was above the Japanese RDA. Furthermore, despite active sunbathing, VD synthesis was insufficient to maintain good health for children with severe disabilities admitted to our institution.
The major sources of VD are dietary intake and cutaneous synthesis via UV exposure. Dietary intake traditionally plays a relatively minor role [9].
However, the extent of cutaneous synthesis and appropriate amounts of VD intake from food required to maintain an adequate VD status for children with severe disabilities who have been institutionalized remain unknown.
We assessed the correlation between VD intake and VD status among children with severe disabilities who had limited UV exposure. We found almost all patients were VD deficient, even though about half had VD intake above the RDA in Japan.
In this study, the serum 25(OH)D levels increased to more than double in 13 patients in group B who had no UV exposure within 15 days before phase 1 and who had at least one UV exposure within 15 days before phase 2, even without a significant increase in dietary intake. Hence, cutaneous synthesis is a very important source of VD in the body of institutionalized children with severe disabilities.
The VD intake of patient no. 31 in group C increased from phase 1 to 2, because the overall diet was increased to improve nutritional status. However, the reason for the significant improvement in VD status without UV exposure was unclear.
In Tokyo, in May, the median serum 25(OH)D levels for 12- to 18-year-olds without UV exposure limitations were 23.56 ng/mL for boys and 20.28 ng/mL for girls. VD intake was 10 μg/day with no difference between boys and girls [10]. The median serum 25(OH)D level in this study was 11.5 ng/mL in phase 1 (May to June) group A, who had sun exposure before blood testing. The median serum 25(OH)D level for all children in phase 1 was 9.15 ng/mL. We found an obvious difference in VD status between children who were institutionalized and those who were not.
In high-latitude areas in Japan and in winter, synthesis in the skin cannot occur even with sunlight exposure. It takes 22.4 min in Tsukuba City, located at latitude 36°N, and 76.4 min in Sapporo, located at latitude 43°N, to synthesize 5.5 μg of VD on 600 cm2 of skin (equivalent to the face and back of both hands of an adult weighing 70 kg) at 0:00 PM in December [11].
Even at our facility, which is located at 39°36′N, VD synthesis via sunbathing in winter is not expected. In particular, among children who require constant ventilator management, sunbathing may be impractical.
Eleven of the 14 patients in group A phase 1 and 19 of 27 patients in group A/B phase 2 had VD intake above the RDA. However, serum 25(OH)D levels exceeded 20 ng/mL in only 1 of 14 patients in group A phase 1 and 10 of 27 patients in group A/B phase 2. These findings suggest that sunbathing significantly affects VD status in institutionalized children with severe disabilities, and that VD deficiency occurs despite VD intake above the RDA. The RDA for VD in Japan is determined by subtracting the amount of synthesis via UV exposure from the US/Canadian standard, which does not take UV exposure into account [6]. Table 2 shows the total VD intake for each menu item according to the dietary standards of our institution and Table 3 depicts the VD content of the blended diet, liquid enteral nutrition formula, and infant formula used at our institution. Children who can take age-appropriate food orally generally meet the recommended intake levels set by the Ministry of Health, Labour and Welfare, but some patients in our study could not take age-appropriate food. In this study, some patients were treated with phenobarbital and valproic acid. The difficulty of UV exposure and the use of antiepileptic drugs that affect the VD status were not taken into account. It is not suitable to apply this RDA to children who are institutionalized.
Considering the target of VD supplementation and its dose, adverse events must be considered. In the general populations, VD supplementation (at doses 5–50 µg/day) probably makes negligible to no contribution in the development of hypercalciuria. Additionally, there is no clear evidence that this amount of VD supplementation is a risk for hypercalcemia. This amount of VD supplementation in children is considered safe [12].
However, VD supplements were considered unsafe for patients with idiopathic hypercalciuria and urinary tract stones because of new urinary tract stone formation [13]. There was a patient who had been diagnosed with urinary tract stones in this study, but it was reported that supplementation with VD (10–20 μg/day) in children with idiopathic hypercalciuria did not affect the serum calcium concentration or urinary calcium excretion and development of urinary tract stones. This VD supplement concentration could be safely administered to children with severe disabilities.
This study was conducted under restrictions on going out and attending school due to the COVID-19 pandemic. VD sufficiency has been shown to play an important role in increasing resistance to viral infection and helping prevent the severe symptoms of COVID-19 that result in fatalities [14].
VD supplementation is necessary not only in the winter but also across all seasons for children with severe disabilities who are institutionalized.
The current study had several limitations. For example, the number of patients is limited, and the length and timing of sun exposure were not appropriately controlled. Hence, future studies should assess VD supplementation among children who cannot sunbathe. Moreover, the trend in serum 25(OH)D levels must be assessed to determine the recommended intake without sunbathing. In conclusion, the findings suggest that Japanese children with severe disabilities who are institutionalized with the recommended VD intake but who have limited UV exposure may still present with VD deficiency.
UV exposure significantly impacts the VD status of Japanese children with severe disabilities. VD supplementation should be considered when UV exposure is not expected to generate sufficient VD due to the need of ventilator management, season, or low latitude.
Data availability
Data supporting the findings of this study are available upon request from the corresponding author. Data were not made publicly available due to privacy or ethical restrictions.
References
Sakai T (2019) Osteoporosis and fractures in patients with severe motor and intellectual disabilities (in Japanese). Nihon Jushoshinshinshogai Gakkaishi (Sev Mot J Intellect Disabil) 44:99–104
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264
Wagner CL, Greer FR, American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition (2008) Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122:1142–1152
Holick MF (2007) Vitamin D deficiency. New Engl J Med 357:266–281
Viraraghavan VR, Seth A, Aneja S, Singh R, Dhanwal D (2019) Effect of high dose vitamin D supplementation on vitamin D nutrition status of pre-pubertal children on anti-epileptic drugs—a randomized controlled trial. Clin Nutr ESPEN 29:36–40
Dietary reference intakes for Japanese (2020) Available from: MHLW.GO.JP. PDF on the internet. Available from: https://www.mhlw.go.jp/content/10904750/000586553.pdf. Accessed 5 Oct 2021
Okazaki R, Ozono K, Fukumoto S, Inoue D, Yamauchi M, Minagawa M, Michigami T, Takeuchi Y, Matsumoto T, Sugimoto T (2017) Assessment criteria for vitamin D deficiency/insufficiency in Japan: proposal by an expert panel supported by the research program of intractable diseases, ministry of health, labour and welfare, Japan, the Japanese society for bone and mineral research and the Japan endocrine society [opinion]. J Bone Miner Metab 35:1–5
Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I (2014) 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab 99:3373–3381
Pilz S, März W, Cashman KD, Kiely ME, Whiting SJ, Holick MF, Grant WB, Pludowski P, Hiligsmann M, Trummer C, Schwetz V (2018) Rationale and plan for vitamin D food fortification: a review and guidance paper. Front Endocrinol 9:373
Tsugawa N, Uenishi K, Ishida H, Ozaki R, Takase T, Minekami T, Uchino Y, Kamao M, Okano T (2016) Association between vitamin D status and serum parathyroid hormone concentration and calcaneal stiffness in Japanese adolescents: sex differences in susceptibility to vitamin D deficiency. J Bone Miner Metab 34:464–474
Miyauchi M, Hirai C, Nakajima H (2013) The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J Nutr Sci Vitaminol 59:257–263
Huey SL, Acharya N, Silver A, Sheni R, Yu EA, Peña-Rosas JP, Mehta S (2020) Effects of oral vitamin D supplementation on linear growth and other health outcomes among children under five years of age. Cochrane Database Syst Rev 12:CD012875
Milart J, Lewicka A, Jobs K, Wawrzyniak A, Majder-Łopatka M, Kalicki B (2020) Effect of vitamin D treatment on dynamics of stones formation in the urinary tract and bone density in children with idiopathic hypercalciuria. Nutrients 12:2521
Benskin LL (2020) A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front Public Health 8:513
Acknowledgements
We are deeply grateful to the nurses at Iwate Prefectural Rehabilitation and Nursery Center for Disabled Children for their support in data collection. This work was not supported by any financial Grants or other funding sources. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
YS designed and performed the study, obtained results, analyzed data, and wrote the manuscript; AK supervised this study; HT and FE helped in performing the study; and TK supervised this study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The current study was approved by the Ethics Committee of Iwate Prefectural Rehabilitation and Nursery Center for Disabled Children, and conducted in accordance with the principles of the Declaration of Helsinki.
Informed consent
Informed consent for the publication of clinical data was obtained in advance from the guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sato, Y., Kamei, A., Toda, H. et al. Vitamin D deficiency in children with severe disabilities under limited ultraviolet exposure. J Bone Miner Metab 41, 52–60 (2023). https://doi.org/10.1007/s00774-022-01376-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01376-w