Abstract
Small neuropeptides from the corpora cardiaca are responsible in cockroaches for the mobilisation of trehalose from the fat body into the haemolymph. Such hypertrehalosaemic hormones (HrTHs) belong to the large family of insect adipokinetic hormones (AKHs); a few HrTHs were previously sequenced from cockroaches, and from genomic and/or transcriptomic information one may predict the genes encoding HrTHs from more species. Definite elucidation of the primary structure of the mature peptide with putative modifications needs analytical chemical methods. In the current study, we use high-resolution mass spectrometry coupled with liquid chromatography to identify unequivocally the HrTHs of 13 cockroach species. Either genomic/transcriptomic information was available for most of the species examined, or from related species. We confirm predicted novel sequences and find hydroxyproline modification for the majority of the peptides. The novel decapeptides are structurally close to Bladi-HrTH, which is found in all seven of the investigated blaberid subfamilies. Bladi-HrTH and all the novel peptides elicit a hypertrehalosaemic response in Periplaneta americana, a blattid cockroach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of a factor in the corpora cardiaca (CC) of the American cockroach Periplaneta americana that was able to elevate haemolymph sugar levels was first published by Steele (1961). The factor was called “hyperglycaemic” but later rather termed “hypertrehalosaemic factor or hormone” (see Goldsworthy and Gäde 1983), because it is the disaccharide trehalose that is the main circulating sugar in most insects. Indeed, an increase of trehalose was shown in P. americana upon hormonal injection by means of isocratic high-performance liquid chromatography (HPLC) on an amino phase support and refractor index detection (Gäde 1991a). Various attempts were made by different research groups to isolate hypertrehalosaemic hormone (HrTH) of this blattid cockroach (see Goldsworthy and Gäde 1983), and in 1984, two absorbance peaks with hypertrehalosaemic activity were separated from extracts of P. americana CC, and the amino acid composition of the respective octapeptides was determined (Gäde 1984, 1985a). Parallel to these studies, peptides with identical amino acid composition had been isolated from P. americana CC using a skeletal muscle bioassay to guide the separation (O’Shea et al. 1984). The myoactive peptides, then called MI and MII, were sequenced (Witten et al. 1984), and the synthetic compounds compared well in HPLC retention time and biological activity with the two hypertrehalosaemic peptides (Gäde 1985b). A third research group came to the same sequence results using a metabolic (= hyperglycaemic) and myotropic (= cardioacceleratory) bioassay during the separation procedure (Scarborough et al. 1984). In 1986, the primary structures for the hypertrehalosaemic peptides of P. americana were confirmed (Siegert and Mordue 1986); today, they are code-named with the acronyms Peram-CAH-I and Peram-CAH-II, with reference to the cardioacceleratory hormone function. In the same year, a decapeptide hormone, now known as Bladi-HrTH, was isolated and sequenced from two blaberid cockroaches Blaberus discoidalis (Hayes et al. 1986) and Nauphoeta cinerea (Gäde and Rinehart 1986; Gäde 1987). In the cockroach Polyphaga aegyptiaca, two octapeptide hypertrehalosaemic peptides were sequenced from the CC: one was previously known to occur in the glands of the tenebrionid beetle Tenebrio molitor (Gäde and Rosinski 1990) and was therefore called Tenmo-HrTH; the second peptide, however, had a unique sequence and is known as Polae-HrTH (Gäde and Kellner 1992). Later, these five peptides were confirmed in species from most taxonomic families of the cockroaches (Gäde 1989; Gäde and Rinehart 1990; Veenstra and Camps 1990; Gäde et al. 1997; König et al. 2005; Predel and Gäde 2005; Roth et al. 2009; Zeng et al. 2021; Jiang et al. 2023).
These HrTHs are members of the well-known adipokinetic hormone (AKH) peptide family. Physiological studies on the effect of HrTHs in cockroaches were mainly performed on P. americana, as well as N. cinerea, B. discoidalis, and Blaptica dubia, and included experiments on the release of the peptides, activation of adenylate cyclase and glycogen phosphorylase, structure–activity relations, and investigations into the influence on the regulation of glycolysis (see, e.g., Gäde 1985c, 1986, 1988, 1991b, 1992; Ford and Hayes 1988; Hayes and Keeley 1990; Sevala and Steele 1991; Gäde and Hayes 1995; Keeley et al. 1996; Becker and Wegener 1998).
As early as 1989 (Gäde 1989), the primary structures of cockroach hypertrehalosaemic peptides have been used to speculate on and/or confirm phylogenetic relatedness of cockroach (order: Blattodea) species. For the first time in 2009, mainly CAPA peptides (insect neuropeptides that are encoded by the capability gene [Kean et al. 2002]), but also hypertrehalosaemic peptides and sulfakinins were used in a proteomic approach to reconstruct the phylogenetic relationships of cockroaches (Roth et al. 2009). A cladogram constructed from the sequence data of these neuropeptides agreed quite well with analyses which took molecular and morphological data into account (for example, Inward et al. 2007; Klass and Meier 2006). The most recent phylogenomic analysis of Blattodea was published by Evangelista et al. 2019. The topologies of that study were mainly confirmed by Bläser et al. (2020) who used the precursor sequences of 17 neuropeptides but not the ones of the AKH peptide family. Recently, Jiang et al. (2023) confirmed, in general, the phylogeny of Blattodea as outlined by Evangelista et al. (2019) with the sequence data of the HrTH receptor, whereas the use of HrTH precursor data did not achieve such a good phylogenetic fit. This most recent study also revealed an early HrTH gene duplication event in the ancestor of one of the superfamilies, the Blaberoidea; based on transcriptomic/genomic data, seven novel sequences are suggested for decapeptides of the AKH peptide family in various blaberoid species.
Bioinformatic predictions, however, are not sufficient to elucidate the structure of a mature AKH. Post-translational modifications are not encoded, and the presence of a gene is not a given fact of expression in the organism. Thus, to arrive at definitive information on the primary sequence of an AKH in any given species, it is imperative to isolate the peptide from the insect and validate its sequence using analytical chemistry methods. In the current study, we used high-resolution mass spectrometry (MS) coupled with nanoflow reversed-phase liquid chromatography (RP-LC) to investigate HrTH peptides from 13 cockroach species. For six species, peptide sequences were available in genomic databases; these were candidates for novel decapeptides. We also included some species with known HrTHs as controls. To examine biological activity of the novel decapeptides, an in vivo metabolic biological assay was performed with adult P. americana specimens as acceptor insect.
Materials and methods
Insects
Adult specimens of various cockroach species were used in this study: the species and their taxonomic affiliations are listed in Table 1; no regard was paid to sex and age of the specimens. All species were purchased from commercial breeders with exceptions. Adult males of the American cockroach, P. americana, were a gift from the research group of Prof. R. Predel (University of Cologne, Germany) and Anaplecta ssp and Xestoblatta cavicola from the research group of Prof. P. Deleporte (University of Rennes, France). Aptera fusca was caught on the premises of the University of Cape Town, South Africa.
The American cockroach was used for biological assays (see below); the animals were held at 25 ± 2 °C, RH at 60%, and 14-h light: 10-h dark cycle and fed with a mixture of dog and rabbit food plus water ad libitum.
Tissue preparation and peptide isolation
CCs were dissected from individual cockroaches of the various species immediately after arrival from the supplier or commercial dealer with the aid of a stereomicroscope at 20- to 40-fold magnification. The glands from the same species were pooled in a microcentrifuge tube containing 80% methanol, extracted by approved methods (Gäde 1984), and dried in a vacuum centrifuge, and aliquots were used in biological assays with the American cockroach and for MS.
Biological assay
The dried methanolic CC extracts were reconstituted in distilled water. Synthetic peptides were dissolved in 40 µL of 50/50 v/v methanol and 0.1% formic acid containing 5% acetonitrile and then diluted 1:1000 with distilled water for injection into P. americana to measure their hypertrehalosaemic activity as previously outlined (Gäde 1980). Briefly, experimentation took place at 25 ± 2 °C; cockroaches were put into individual film canisters with a wad of moist cotton wool and left to rest in the dark for 1 h before 1 µL of haemolymph was withdrawn from the base of a leg with a glass microcapillary. The haemolymph was blown into a glass test tube containing 100 µL sulfuric acid. The animal was then injected into the abdominal cavity with 10 µL of the test solution via a 25 µL Hamilton syringe and returned to rest in the canister for 90 min whereafter a second sample of haemolymph was removed as described above.
The colorimetric measurement of carbohydrates was done according to Holwerda et al. (1977): 1 mL of anthrone solution (120 mg anthrone, 60 mL of H2SO4, 30 mL of distilled water) was added to each test tube, mixed thoroughly, and then heated at 100 °C for 8 min in a heating block. Thereafter, tubes were cooled in cold water for 5 min and incubated in the dark for 30 min, and the samples were measured in a Helios spectrophotometer at a wavelength of 585 nm. The carbohydrate concentration could be gleaned from a calibration curve for carbohydrates using the same methodology with known amounts of glucose.
The difference in carbohydrate concentration before and after injection was calculated for each individual animal, and a paired t-test was used for calculating statistical significance in Excel. An analysis of variance (ANOVA) with Tukey’s HSD (honestly significant difference) test (to control the type I error rate, see Fernandez 1992) was used to test for significant differences between different groups of cockroaches injected with different synthetic peptides at the same dose (10 pmol). Briefly, the distribution of data, including an assessment of normality, was carried out with the UNIVARIATE Procedure to validate the use of ANOVA; the relative per cent change following injection of a substance was calculated by the formula ((T90 – T0) / T0) *100, where the carbohydrate concentration before injection (T0) and 90 min after injection (T90) is considered for each substance. The mean relative per cent change for each group of animals was compared by Tukey’s HSD test (p ≤ 0.05). The normality test, ANOVA, and Tukey’s test were generated using SAS Enterprise Guide v. 8.3 (SAS Institute, Cary, NC, USA).
AKH separation and sequence characterisation by LC–MS
The dried methanolic CC extracts were dissolved in 10 μL methanol followed by 10 μL 0.1% formic acid containing 5% acetonitrile. For LC–MS/MS, Synapt G2 Si (Q-TOF with ion mobility function) coupled to M-Class nano-UPLC (Waters Corp., Manchester, UK) was employed using C18 μPAC columns (trapping and 50 cm analytical; PharmaFluidics, Ghent, Belgium) with a 30 min gradient (10–60%; solvent system 100% water versus 100% acetonitrile, both containing 0.1% formic acid; 1 μL injection volume). AKH candidates were identified by target-MS (MS/MS on pre-selected m/z values) for eligible known peptide masses from related insect species using their singly and doubly charged ions, as well as by screening with low/high collision energy switching for the gas phase loss of the tryptophan immonium ion in data-independent runs. Moreover, AKH candidates were obtained by manual interrogation of data-dependent runs and the use of marker fragment ions discovered for proline-containing AKHs (König et al. 2023).
Sequence ion assignment was used as calculated by the MassLynx spectrometer software, which treats pyroglutamate (Pyr) as terminal modification rather than a modified amino acid, thus creating a label shift for ion assignment by one in comparison to the amino acid number. The fragment ion tables for the spectra shown here are available in the Supplement for clarification. Peptide sequences were validated by comparison to the performance of the respective synthetic peptides; in the case of the hydroxyproline forms where synthetic peptides were mostly not available, the sequences could not be validated and those peptides are considered putative. Both the endogenous and the synthetic samples were spiked with bradykinin 1–7 (Sigma, 1 pmol/µL stock solution) for control of the retention time, which was about 6 min earlier than for the AKHs. It allowed correction of the LC arrival time following heavy unrelated use of the instrumentation, if necessary. The peptides were run with identical parameters separated by blank runs.
Synthetic peptides
The novel adipokinetic peptides elucidated in this study, scrambled Peram-CAH-I, Tenmo-HrTH, and its [Hyp6] form, were purchased from Pepmic Co., Ltd. (Suzhou, China). Peram-CAH-I and Bladi-HrTH were purchased from Peninsula Laboratories (Belmont, CA, USA).
Results
Hypertrehalosaemic activity of cockroach CC extracts
In a first series of experiments, glandular extracts from the various cockroaches under study were tested in mobilising trehalose in American cockroaches. CC material from a subset of cockroaches only was analysed in this study: some we had examined before (S. longipalpa, N. cinerea, Gäde 1989) and are not repeated here, other species were not available in sufficient numbers (Aptera fusca), or not easily obtained. We further selected to study mainly those species of whom we had genomic/transcriptomic data that hinted at novel HrTHs not previously identified in CC extracts.
As summarised in Table 2, 0.2 gland equivalents of the CC were able to elevate the level of total carbohydrates in the haemolymph significantly, whereas control injections with water had no significant effect. The total increase in circulating carbohydrates in P. americana 90 min after injection of the conspecific CC extracts was in the same range (15 to 18 µg/µL) as measured after injection of the positive control, i.e. the synthetic peptide Peram-CAH-I, an endogenous octapeptide of P. americana (Table 2). The effect of the CC extract of A. kyotensis, however, was slightly lower (Table 2), and this may be due to the smaller size of the insect and its CC. Nevertheless, this experiment has clearly demonstrated the presence of hypertrehalosaemic material in the CC of the selected cockroaches.
LC–MS sequence analysis of hypertrehalosaemic peptides from various cockroaches
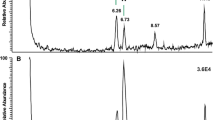
In most of the investigated species, the decapeptide Bladi-HrTH (pQVNFSPGWGT amide) was detected (Table 1; for an exemplary spectrum, from the CC of P. nivea, see Fig. 1) and validated (see Supplementary Information [SI], Figs. S1, S2). Interestingly, for Bladi-HrTH itself, as well as its novel decapeptide relatives (see below), hydroxyproline-containing forms (Hyp) were found in LC and sequenced by MS/MS (Table 1), but in most cases, this was not validated with a synthetic peptide and hence termed here as “putative”. Exemplary spectra of Hyp-modified-HrTHs are shown below for P. nivea and Ergaula capucina.
HYPERLINK "sps:id::fig1||locator::gr1||MediaObject::0"MS target analysis for Bladi-HrTH in Panchlora nivea (for validation, see Supplementary Figure S2A). The singly and the doubly charged ions were fragmented (M + H+ m/z 1074.50, [M + 2H].2+ m/z 537.75). Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S1
The Blaberoidea
Eublaberus posticus, Nauphoeta cinerea, Aptera fusca, Xestoblatta cavicola
For four species of the Blaberidae, Bladi-HrTH, and its Hyp derivative were detected and sequenced (Table 1).
Panchlora nivea
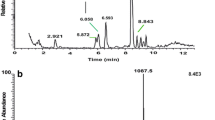
Besides Bladi-HrTH (Fig. 1) and its Hyp derivative, another AKH was detected with a sequence of pQLNFSPGWGT amide (Fig. 2). This novel, predicted peptide, now code-named Panni-HrTH, eluted ~ 2 min later than Bladi-HrTH (33.8 min) in LC and is, structurally, a Leu2 modified version of Bladi-HrTH. The sequence of Panni-HrTH was validated using the synthetic peptide (SI, Fig. S3, S4). A Hyp variant of Panni-HrTH had a retention time of 32.2 min, and a putative sequence was assigned on the basis of MS/MS sequencing (Fig. 3; SI Fig. S5, S6).
MS target analysis for L2 Bladi-HrTH (= Panni-HrTH) in Panchlora nivea (for validation, see Supplementary Figure S4). The singly and the doubly charged ions were fragmented (M + H+ m/z 1088.52, [M + 2H]2+, m/z 544.76). Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S3. This is a novel member of the AKH family
MS/MS analysis for the singly and the doubly charged peptide ions (m/z 1104.51 and 552.76) of a peptide assigned to [Hyp6]-Panni-HrTH in Panchlora nivea CC extract. Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S5. For original spectra, see Figure S6. The shift of 16 Da for the abundant fragment ions around the Pro residue (y”5, y”6, y”7, b7, b8) proves the position of the oxidation on the Pro residue
Blaberus atropos
Bladi-HrTH and a Hyp derivative of Bladi-HrTH were detected in the CC extract of B. atropos (Table 1) along with a novel decapeptide with the primary sequence of pQLNFSPGWGF amide (37.7 min, Fig. 4). This novel sequence was genomically predicted, and the peptide is here given the code-named Blaat-HrTH and validated. Compared with Bladi-HrTH, Blaat-HrTH has a Leu2 and Phe10 modification (Table 1); the sequence was validated with a corresponding synthetic peptide (SI, Fig. S7, S8). A Hyp variant of Blaat-HrTH was also sequenced.
MS target analysis for L2F10 Bladi-HrTH (= Blaat-HrTH) in Blaberus atropos (for validation, see Supplementary Figures S7 and S8). The singly and the doubly charged ions were fragmented (M + H+ m/z 1134.54, [M + 2H]2+ m/z 567.77). Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S7. This is a novel member of the AKH family
Loboptera decipiens and Episymploce sundaica
Identical peptides were found in L. decipiens and E. sundaica: Bladi-HrTH and its Hyp derivative, as well as a novel decapeptide sequence (pQLNFSPGWGP amide, 34.1 min, Fig. 5) and a Hyp variant of the latter (Table 1). The novel sequence was predicted from genomic information and first detected here in L. decipiens; hence, we assign the code-named Lobde-HrTH for the novel peptide that is common to both L. decipiens and E. sundaica. Compared with Bladi-HrTH, Lobde-HrTH has a Leu2 and a Pro10 substitution; the primary sequence was validated (SI, Fig. S9, S10).
MS target analysis for L2P10 Bladi-HrTH (= Lobde-HrTH) in Loboptera decipiens (for validation, see Supplementary Figure S10). The singly and the doubly charged ions were fragmented (M + H+ m/z 1084.52, [M + 2H]2+ m/z 542.76). Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S9. This is a novel member of the AKH family
Asiablatta kyotensis and Supella longipalpa
In both species, Bladi-HrTH and its Hyp derivative were found in the CC extracts, plus a novel decapeptide (pQLNFSPGWGV amide, 35.6 min, Fig. 6; Table 1). This novel peptide was first biochemically demonstrated here in A. kyotensis and is, therefore, given the code-named Asiky-HrTH. The structure was predicted from genomic information and was validated with a corresponding synthetic peptide (SI, Fig. S11, S12). In comparison to Bladi-HrTH, Asiky-HrTH has two modifications (Leu2 and Val10). Curiously, the Hyp variant of Asiky-HrTH was only detected and sequenced from A. kyotensis and not from S. longipalpa CC (Table 1)—this may possibly be due to a low concentration (below detection) in the very tiny CC of S. longipalpa.
MS target analysis for L2V10 Bladi-HrTH (= Asiky-HrTH) in Asiablatta kyotensis (for validation, see Supplementary Figure S12). The singly and the doubly charged ions were fragmented (M + H+ m/z 1086.54, [M + 2H]2+ m/z 543.77). Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S11. This is a novel member of the AKH family
The Solumblattodea
Periplaneta americana, Anaplecta ssp, and Ergaula capucina
P. americana is known to contain the two hypertrehalosaemic octapeptides Peram-CAH-I (pQVNPSPNW amide) and Peram-CAH-II (pQLTFTPNW amide) (see Introduction for references; SI, Fig. S13). We also detected these two octapeptides (32.1 min, 34.9 min) and show the spectra in Supplementary Fig. S14. In Anaplecta ssp, only Peram-CAH-I was found (SI, Fig. S14), while E. capucina presented the well-known octapeptide Tenmo-HrTH (pQLNFSPNW amide) (SI, Fig. S15, S16) and the Hyp form of Tenmo-HrTH (Fig. 7). [Hyp6]-Tenmo-HrTH was validated with a synthetic peptide of the same sequence (SI, Fig. S17, S18). The shift of 16 Da for the abundant fragment ions around the Pro residue (y”3, y”4, y”5, y”6, b4) proves the position of the oxidation on the Pro residue.
MS/MS analysis for [Hyp6]-Tenmo-HrTH in Ergaula capucina using the singly-charged peptide ion (m/z 1003.46). Peaks were labelled according to the b- and y-ion series as calculated in Supplementary Figure S17. The shift of 16 Da for the abundant fragment ions around the Pro residue (y”3, y”4, y”5, y”6, b4) proves the position of the oxidation on the Pro residue. For original spectrum and validation with the synthetic compound, see Figure S18. Inset: overview spectrum showing the protonated and sodiated peptide peaks
Biological activity of the novel hypertrehalosemic peptides
Once the novel HrTHs were chemically identified, they were commercially synthesised and tested in a heterospecific in vivo biological assay at a concentration of 10 pmol in American cockroaches. An additional octapeptide was synthesised and tested: the peptide has the same amino acid residues as Peram-CAH-I but with a scrambled location of the aromatic amino acids (i.e. Trp4 and Phe8 instead of Phe4 and Trp8). Table 3 compares the results of the four novel HrTHs with two positive controls (the endogenous octapeptide Peram-CAH-I and the known blaberid decapeptide Bladi-HrTH) and with two negative controls (water and the Trp4Phe8-Peram-CAH-I analogue). Injections of the two negative controls had no significant effect on trehalose release from the fat body into the haemolymph, in contrast to all other peptides. The individual hypertrehalosaemic response was calculated as per cent relative change in each group of cockroaches, and the means were subjected to ANOVA analyses with Tukey’s HSD test to interrogate statistically significant differences (Table 3): although there is a clear ranking of the relative biological response from the highest (Peram-CAH-I) to the lowest (Blaat-HrTH) with the cockroach decapeptides tested, these differences are not statistically significant.
Discussion
A recent survey of genomic/transcriptomic information in publicly accessible databases, for the purposes of phylogenetic analyses based on AKH precursor sequences and AKH receptor sequences in the order Blattodea (termites and cockroaches), revealed seven preprohormone sequences that predicted novel AKH peptides in cockroaches (Jiang et al. 2023). One of the major objectives of the current study was to clarify with sound chemical methods the primary structure of those predicted AKH family peptides and to ascertain the physiological role of those predicted peptides as hypertrehalosaemic hormones (HrTHs) in a well-studied cockroach species, Periplaneta americana. We were able to source cockroach species in which four out of the seven peptides should occur. Unfortunately, the remaining three predicted decapeptides were proposed to occur in species that are not in culture in any laboratory, hence not easy to obtain for validating the predicted structures. Due to the key structural features of members of the AKH peptide family, such as the post-translationally modified termini (a pyroglutamic acid at the N-terminus and a carboxyamide at the C-terminus), it is not clear from molecular sequences alone whether during the biosynthesis process of the large precursor peptide, the resulting mature peptide will display the typical AKH family characteristics, or whether the dibasic splicing site is cleaved differently, thereby resulting in a longer C-terminally unblocked entity with negative consequences for functional activation of the AKH receptor (Marco et al. 2022). Likewise, the presence of post-translational modifications of non-terminal amino acids of the AKH/HrTH peptide is potentially possible and not predictable at this stage, for example C-Trp mannosylation (Gäde et al. 1992; Munte et al. 2008), Thr phosphorylation (Gäde et al. 2006), hydroxyprolination (Gäde et al. 2011), or Thr sulphation (Gäde et al. 2014).
The MS measurements of the current study gave clear answers to the questions of the structure of four of the predicted novel peptides, which we have now code-named Panni-HrTH (in Panchlora nivea), Blaat-HrTH (in Blaberus atropos), Lobde-HrTH (in Loboptera decipiens and Episymploce sundaica), and Asiky-HrTH (in Asiablatta kyotensis and Supella longipalpa). We could neither confirm nor refute the primary structure of the three remaining novel peptides as we do not have access to the cockroach species in which the peptides were predicted from transcriptomes, viz. in Cariblatta sp. and Nyctibora sp. (Jiang et al. 2023). All 13 investigated cockroach species in the current study produce HrTHs that have the characteristic post-translationally modified termini of this peptide family. Furthermore, the peptides are correctly cleaved at the dibasic splicing site. The MS technique also revealed unequivocally that the genomically/transcriptomically predicted forms of HrTHs in the investigated blaberoid cockroaches are indeed expressed and confirmed the sequences. Validation of the natural and synthetic compounds by retention time and CID pattern resulted always in a perfect match. Thus, there is no doubt that certain blaberid cockroaches produce two decapeptides in their CC, both of which are very similar in primary structure and could have evolved from each other. A hypothetical scheme for molecular evolution of all Blattodea HrTHs is given in Fig. 8. It is noteworthy that all decapeptides, including the three that are still only predicted (see Fig. 8), are on one side of the scheme and can evolve from each other with only point mutations. Only in one case of the Blattodea HrTH peptides, involving the octapeptides Peram-CAH-I and Manto-CC, would two nucleotides of the triplet have to mutate in this hypothetical evolutionary scheme.
Hypothetical molecular evolution of adipokinetic peptides in Blattodea. Peram-CAH-I is assumed as ancestral peptide for this order. The amino acid substitution in each peptide is indicated in a bold larger font than the peptide from which it is hypothetically derived. All substitutions are point mutations except the change from Peram-CAH-I to Manto-CC, where the switch from Asn7 to Gly7 requires two base changes. Note that three hitherto unconfirmed novel decapeptides (= Predicted, from data mining databases of Cariblatta sp. and Nyctibora sp.; see Jiang et al. 2023) are included in the scheme
In the majority of cockroach samples analysed in the current study, the crude extracts of corpora cardiaca displayed an additional mass signal that when sequenced, corresponded to the hypertrehalosaemic peptide(s) of that species with a hydroxyproline residue at position 6; we have validated only one of these sequences ([Hyp6]-Tenmo-HrTH, while the other “putatives” remain to be validated. In insects, hydroxyprolination of an AKH was first shown in the green stink bug Nezara viridula (order Hemiptera, suborder Heteroptera) as an analogue of the octapeptide Panbo-RPCH (Gäde et al. 2011), and recently for Tabat-AKH in the horse fly Haematopota pluvialis (Haepl-AKH; Marco et al. 2022). In N. viridula, it was shown that Nezvi-AKH—the peptide with the hydroxyproline in position 6—was a genuine occurrence and not merely an artefact; furthermore, this atypical AKH was biologically active in lipid mobilisation in the stink bug, as was its Pro analogue, Panbo-RPCH (Gäde et al. 2011). Possibly, hydroxyprolination of AKHs is more common than previously thought, and the enigma of whether this modification may be a natural phenomenon in the CC, or an artefact arising during peptide handling will require further investigation.
Another objective of this study was to show that the novel decapeptides have biological, thus trehalose mobilising activity in cockroaches. In most of the investigated species, the decapeptide Bladi-HrTH (pQVNFSPGWGT amide) was detected and validated (Table 1), adding to the body of information that this is a hallmark peptide of blaberid cockroaches (see, e.g., Hayes et al. 1986; Gäde and Rinehart 1986; Roth et al. 2009; Jiang et al. 2023). Although the American cockroach is a blattid cockroach, we nevertheless selected it as acceptor insect for in vivo functional assays, for two reasons: haemolymph of P. americana does not clot easily, and there is a plethora of physiological information on this species, including structure–activity studies with the AKH family of peptides (see Introduction). P. americana synthesises two octapeptides, but its AKH receptor also recognises the decapeptide Bladi-HrTH very well: whereas the endogenous hormones Peram-CAH-I and Peram-CAH-II had ED50 values (the amount of peptide needed to produce 50% of the maximal possible response) between 0.5 and 1 pmol, a maximum response was achieved with 4 pmol, the ED50 value for Bladi-HrTH was between 1.6 and 3 pmol, and 10 pmol were needed for a maximum response (Gäde 1990; Gäde and Hayes 1990; Table 3). The first six amino acids of Bladi-HrTH are identical with those of Peram-CAH-I with a difference occurring at position 7, viz. a simple glycine residue instead of the asparagine of the P. americana octapeptide (see Table 1 for structures). Peram-CAH-II, the second HrTH octapeptide of P. americana on the other hand, registers three differences in the first six amino acids compared with Peram-CAH-I and Bladi-HrTH and still elicited a 100% hypertrehalosaemic response in P. americana (Gäde 1990). The novel cockroach decapeptides validated in this study may be described as analogues of Bladi-HrTH for they differ from Bladi-HrTH in only one or two positions; they are also able to activate the P. americana HrTH receptor (Table 3). The leucine at position 2 (Panni-HrTH) is well tolerated, which is in line with a leucine residue at this position in the second P. americana octapeptide.
A double substitution compared to Bladi-HrTH, leucine at position 2 plus either valine, proline or phenylalanine at position 10 instead of threonine produced smaller trehalosaemic responses than measured with Bladi-HrTH and Panni-HrTH but did not differ significantly from the increase resulting from Peram-CAH-I (Table 3). It would seem, thus, that although the P. americana HrTH receptor favours octapeptides, decapeptides can bind and influence the biological activity in this blattid cockroach. Structure–activity studies have also been reported for B. discoidalis with Bladi-HrTH analogues (Ford and Hayes 1988; Hayes and Keeley 1990): Ala2-Bladi-HrTH and Ala10-Bladi-HrTH had small or no influence on activity. Thus, it is very likely that the novel peptides which were confirmed by MS methods in this study may also have no or only little influence on binding to the B. discoidalis HrTH receptor and will perform with maximal activity in blaberid cockroaches.
Ideally, the novel peptides should also be tested conspecifically. This, however, is not such a simple task, because the haemolymph of blaberid cockroaches coagulates immediately when in contact with air. A method is described and evaluated to determine trehalose in the coagulum of B. discoidalis (Keeley et al. 1991; Sowa and Keeley 1996). If this method can be modified for small species of the genera Episymploce, Asiablatta, Supella and Panchlora remains to be seen.
Lastly, this study may also have helped in the discussion of phylogenetic placement of certain cockroach families. Whereas Legendre et al. (2015) have Anaplecta in the superfamily Blaberoidea nested within Ectobiidae as sister taxon to several Pseudophyllodromiinae, Djernæs et al. (2020) have this species and family Anaplectidae in the superfamily Blattoidea close to the clades Tryonicidae, Lamproblattidae, and Cryptocercidae plus Isoptera (termites). Our finding of Peram-CAH-I in Anaplecta is consistent with the latter view.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- HrTH:
-
Hypertrehalosaemic hormone
- AKH:
-
Adipokinetic hormone
- CC:
-
Corpora cardiaca
- HPLC:
-
High-performance liquid chromatography
- MS:
-
Mass spectrometry
- RP-LC:
-
Reversed-phase liquid chromatography
References
Becker A, Wegener G (1998) Hypertrehalosaemic neuropeptides decrease levels of the glycolytic signal fructose 2,6-bisphosphate in cockroach fat body. J Exp Biol 201:1939–1946
Bläser M, Misof B, Predel R (2020) The power of neuropeptide precursor sequences to reveal phylogenetic relationships in insects: a case study on Blattodea. Mol Phylogenetics Evol 143:533
Djernæs M, Varadínová ZK, Kotyk M, Eulitz U, Klass K-D (2020) Phylogeny and life history evolution of Blaberoidea (Blattodea). Arthropod Syst Phylogeny 78:29–67
Evangelista DA, Wipfler B, Bethoux O, Donath A, Fujita M, Kohli MK et al (2019) An integrative phylogenomic approach illuminates the evolutionary history of cockroaches and termites (Blattodea). Proc Royal Soc b 286:20182076. https://doi.org/10.1098/rspb.2018.2076
Fernandez GCJ (1992) Residual analysis and data transformations: important tools in statistical analysis. HortScience 27(4):297–300
Ford M, Hayes T, Keeley L (1988) Structure-activity relationships for insect hypertrehalosaemic hormone: the importance of side chains and termini. In: Marshall GR (ed) Peptides chemistry and biology. ESCOM, The Netherlands, pp 653–655
Gäde G (1980) Further characteristics of adipokinetic and hyperglycaemic factor(s) of stick insects. J Insect Physiol 26:351–360
Gäde G (1984) Adipokinetic and hyperglycaemic factors of different insect species: Separation with high-performance liquid chromatography. J Insect Physiol 30:729–736
Gäde G (1985a) Amino acid composition of cockroach hypertrehalosaemic hormones. Z. Naturfosch. 40c:42–46
Gäde G (1985b) Hypertrehalosaemic hormones and myoactive factors from cockroach corpus cardiacum are very likely identical. Naturwiss 72:95–96
Gäde G (1985c) Mode of action of the hypertrehalosaemic peptides from the American cockroach. Z Naturforsch 40c:670–676
Gäde G (1986) Relative hypertrehalosaemic activities of naturally occurring neuropeptides from the AKH/RPCH family. Z Naturforsch 41c:315–320
Gäde G (1987) Characterization and amino acid composition of a hypertrehalosaemic neuropeptide from the corpora cardiaca of the cockroach, Nauphoeta cinerea. Z Naturforsch 42c:225–230
Gäde G (1988) On the release and action of the hypertrehalosaemic hormone from the cockroach Nauphoeta cinerea. Z Naturforsch 43c:108–116
Gäde G (1989) The hypertrehalosaemic peptides of cockroaches: a phylogenetic study. Gen Comp Endocrinol 75:287–300
Gäde G (1990) Structure-function studies on hypertrehalosaemic and adipokinetic hormones: activity of naturally occurring analogues and some N- and C-terminal modified analogues. Physiol Entomol 15:299–316
Gäde G (1991a) Hyperglycaemia or hypertrehalosaemia? The effect of insect neuropeptides on haemolymph sugars. J Insect Physiol 37:483–487
Gäde G (1991b) Glycogen phosphorylase in the fat body of two cockroach species, Periplaneta americana and Nauphoeta cinerea: isolation, partial characterization of three forms and activation by hypertrehalosaemic hormones. Z Naturforsch 46c:149–162
Gäde G (1992) Structure-activity relationships for the carbohydrate-mobilising action of further bioanalogues of the adipokinetic hormone/ red pigment-concentrating hormone family of peptides. J Insect Physiol 38:259–266
Gäde G, Rinehart KL (1986) Amino acid sequence of a hypertrehalosaemic neuropeptide from the corpus cardiacum of the cockroach, Nauphoeta cinerea. Biochem Biophys Res Commun 141:774–781
Gäde G, Hayes TK (1990) Insect hypertrehalosemic hormone: structure-activity studies in Periplaneta americana. In: Borkovec AB, Masler EP (eds) Insect Neurochemistry and Neurophysiology. The Humana Press, Clifton
Gäde G, Rinehart KL (1990) Primary structures of hypertrehalosaemic neuropeptides isolated from the corpora cardiaca of the cockroaches Leucophaea maderae, Gromphadorhina portentosa, Blattella germanica and Blatta orientalis and of the stick insect Extatosoma tiaratum assigned by tandem fast atom bombardment mass spectrometry. Biol Chem Hoppe-Seyler 371:345–354
Gäde G, Rosinski G (1990) The primary structure of the hypertrehalosaemic neuropeptide from tenebrionid beetles: a novel member of the AKH/RPCH family. Peptides 11:455–459
Gäde G, Kellner R (1992) Primary structures of the hypertrehalosemic peptides from corpora cardiaca of the primitive cockroach Polyphaga aegyptiaca. Gen Comp Endocrinol 86:119–127
Gäde G, Hayes TK (1995) Structure-activity relationships for Periplaneta americana hypertrehalosemic hormone I: the importance of side chains and termini. Peptides 16:1173–1180
Gäde G, Kellner R, Rinehart KL, Proefke ML (1992) A tryptophan-substituted member of the AKH/RPCH family isolated from a stick insect corpus cardiacum. Biochem Biophys Res Commun 189:1303–1309
Gäde G, Grandcolas P, Kellner R (1997) Structural data on hypertrehalosaemic neuropeptides from Cryptocercus punctulatus and Therea petiveriana: how do they fit into the phylogeny of cockroaches? Proc R Soc Lond B 264:763–768
Gäde G, Šimek P, Clark KD, Auerswald L (2006) Unique translational modification of an invertebrate neuropeptide: a phosphorylated member of the adipokinetic hormone peptide family. Biochem J 393:705–713
Gäde G, Šimek P, Marco HG (2011) An invertebrate [hydroxyproline]-modified neuropeptide: further evidence for a close evolutionary relationship between insect adipokinetic hormone and mammalian gonadotropin hormone family. Biochem Biophys Res Commun 414:592–597
Gäde G, Šimek P, Marco HG. A sulfothreonine adipokinetic peptide—a novel post-translational modification revealed in the twig wilter Holopterna alata (Hemiptera, Coreidae). In: 27th Conference of European Comparative Endocrinologists, 2014, OR 26.
Goldsworthy GJ, Gäde G (1983) The chemistry of hypertrehalosemic factors. In: Downer RGH, Laufer H (eds) Endocrinology of Insects. A.R. Liss Inc., New York, pp 109–119
Hayes TK, Keeley LL (1990) Structure-activity relationships on hyperglycemia by representatives of the adipokinetic/hyperglycemic hormone family in Blaberus discoidalis cockroaches. J Comp Physiol 160:187–194
Hayes TK, Keeley LL, Knight DW (1986) Insect hypertrehalosaemic hormone: Isolation and primary structure from Blaberus discoidalis cockroaches. Biochem Biophys Res Commun 140:674–678
Holwerda DA, Doorn J, Beenakkers AMT (1977) Characterization of the adipokinetic and hyperglycaemic substances from the locust corpus cardiacum. Insect Biochem 7:151–157
Inward D, Beccaloni G, Eggleton P (2007) Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol Lett 3:331–335
Jiang S, Marco HG, Scheich N, He S, Wang Z, Gäde G et al (2023) Comparative analysis of adipokinetic hormones (AKHs) and their receptors (AKHRs) in Blattodea reveals novel patterns of gene evolution. Insect Mol Biol. https://doi.org/10.1111/imb.12861
Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP et al (2002) Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am J Physiol Regul Integr Comp Physiol 282:R1297-1307
Keeley LL, Hayes TK, Bradfield JY, Sowa SM (1991) Physiological actions by hypertrehalosemic hormone and adipokinetic peptides in adult Blaberus discoidalis cockroaches. Insect Biochem 21:121–129
Keeley LL, Park JH, Lu K-H, Bradfield JY (1996) Neurohormone signal transduction for dual regulation of metabolism and gene expression in insects: hypertrehalosemic hormone as a model. Arch Insect Biochem Physiol 33:283–301
Klass KD, Meier R (2006) A phylogenetic analysis of Dictyoptera (Insecta) based on morphological characters. Entomol Abh 63:3–50
König S, Albers C, Gäde G (2005) Mass spectral signature for insect adipokinetic hormones. Rapid Commun Mass Spectrom 19:3021–3024
König S, Marco HG, Gäde G (2023) The proline effect and the tryptophan immonium ion assist in de novo sequencing of adipokinetic hormones. Sci Rep 13(1):10894. https://doi.org/10.1038/s41598-023-38056-2
Legendre F, Nel A, Svenson GJ, Robillard T, Pellens R, Grandcolas P (2015) Phylogeny of Dictyoptera: dating the origin of cockroaches, praying mantises and termites with molecular data and controlled fossil evidence. PlosOne 10:e0130127
Marco HG, König S, Gäde G (2022) Mass spectrometric proof of predicted peptides: Novel adipokinetic hormones in insects. Molecules 27:6469
Munte CE, Gäde G, Domogalla B, Kremer W, Kellner R, Kalbitzer HR (2008) C-mannosylation in the hypertrehalosaemic hormone from the stick insect Carausius morosus. FEBS 275:1163–1173
O’Shea M, Witten J, Schaffer M (1984) Isolation and characterization of two myoactive neuropeptides: further evidence of an invertebrate peptide family. J Neurosci 4:521–529
Predel R, Gäde G (2005) Peptidomics of neurohemal organs from species of the cockroach family Blattidae: how do neuropeptides of closely related species differ? Peptides 26:3–9
Roth S, Fromm B, Gäde G, Predel R (2009) A proteomic approach for studying insect phylogeny: CAPA peptides of ancient insect taxa (Dictyoptera, Blattoptera) as a test case. BMC Evol Biol 9:50
Scarborough RM, Jamieson GC, Kalish F, Kramer SJ, McEnroe GA, Miller CA et al (1984) Isolation and primary structure of two peptides with cardioacceleratory and hyperglycemic activity from the corpora cardiaca of Periplaneta americana. Proc Natl Acad Sci USA 81:5575–5579
Sevala VL, Steele JE (1991) Regulation of glycolytic intermediates in cockroach fat body by the corpus cardiacum. J Comp Physiol B 161:349–355
Siegert KJ, Mordue W (1986) Elucidation of the primary structures of the cockroach hyperglycaemic hormones I and II using enzymatic techniques and gas-phase sequencing. Physiol Entomol 11:205–211. https://doi.org/10.1111/j.1365-3032.1986.tb00407.x
Sowa SM, Keeley LL (1996) Free amino acids in the hemolymph of the cockroach, Blaberus discoidalis. Comp Biochem Physiol 113A:131–134
Steele JE (1961) Occurrence of a hyperglycemic-factor in the corpus cardiacum of an insect. Nature 192:680–681
Veenstra JA, Camps F (1990) Structure of the hypertrehalosemic neuropeptide of the German cockroach Blattella germanica. Neuropeptides 15:107–109
Witten JL, Schaffer MH, O’Shea M, Cook JC, Hemling ME et al (1984) Structures of two cockroach neuropeptides assigned by fast atom bombardment mass spectrometry. Biochem Biophys Res Commun 124:350–358
Zeng HC, Qin YR, Du EX, Wei QL, Li Y, Huang DY et al (2021) Genomics- and peptidomics-based discovery of conserved and novel neuropeptides in the American cockroach. J Proteome Res 20:1217–1228
Acknowledgements
The authors are grateful to various colleagues for the gift of certain insects (see Materials and Methods). GG and HGM wish to thank Prof. Dr. Bettina Zeis (Institut für Integrative Zellbiologie und Physiologie, Universität Münster, Germany) for support with laboratory space and usage of certain instruments and Dr Paulo Cremonez (Department of Entomology, University of Georgia, USA) for statistical advice.
Funding
Open access funding provided by University of Cape Town. This research was funded in part by the National Research Foundation of South Africa (Grant No 85768 [IFR13020116790] to GG and grant no 10924 to HGM). Moreover, the Research Council of the University of Cape Town funded GG and HGM through staff awards.
Author information
Authors and Affiliations
Contributions
All authors have given approval to the final version of the manuscript. GG sourced animals and performed bioassays and HGM performed CC excision and extraction, as well as bioassays, data analyses, and interpretation. SK conducted the MS experiments and data analyses of the methanolic extracts as well as de novo sequencing. GG helped with the data interpretation. HGM and GG conceptualised the project; HGM, GG, and SK designed the methodology; HGM, GG, and SK involved in formal analysis during the investigation; GG and SK prepared the original draft; HGM, GG, and SK wrote, reviewed, and edited subsequent drafts; HGM and GG acquired the funding; HGM, GG, and SK gathered the resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The authors have no relevant financial or non-financial interests to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling editor: E. Closs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marco, H.G., König, S. & Gäde, G. Predicted novel hypertrehalosaemic peptides of cockroaches are verified by mass spectrometry. Amino Acids 55, 1641–1654 (2023). https://doi.org/10.1007/s00726-023-03337-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-023-03337-7