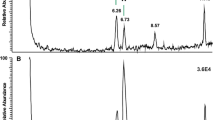
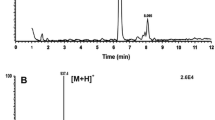
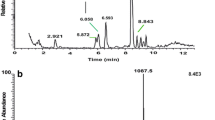
Summary
Several members of the adipokinetic/hyperglycemic neurohormone family from several different invertebrate species have been prepared by solid-phase peptide synthesis and assayed by a modified in vivo hyperglycemic bioassay in Blaberus discoidalis cockroaches. The hypertrehalosemic hormone (HrTH) is the endogenous hypertrehalosemic factor for B. discoidalis and was the most potent peptide in the assay. The more divergent the sequence of a family member from Blaberus HrTH, the less potent was the bioanalog. Manduca adipokinetic hormone is the most divergent peptide of the family and was totally inactive in the bioassay. Locusta adipokinetic hormone I had reduced maximum activity in the assay, which suggests that Ser5 is an important residue for the transduction of the hyperglycemic response. The direct relation between bioanalog similarity to Blaberus HrTH sequence and potency suggests that the hormone and target cell receptor for HrTH have evolved to maintain an “optimal fit”.
Similar content being viewed by others
Abbreviations
- AKH :
-
adipokinetic hormone
- HrGH :
-
hyperglycemic hormone
- HrTH :
-
hypertrehalosemic hormone
- RPCH :
-
red pigmentconcentrating hormone
- CAH:
-
cardioacceleratory hormone. Hormone abbreviations are according to the convention of Gäde and Raina (1989) except that the genus names are not abbreviated
References
Baniak EL, Gierasch LM, Rivier J, Hagler AT (1988) NMR analysis and conformational characterization of fat cyclic antagonists of gonadotropin-releasing hormone. In: Peptides, Chemistry and Biology (G.M. Marshall, ed.) 457–458
Bowers WS, Friedman S (1963) Mobilization of body glycogen by an extract of corpus cardiacum. Nature 198:685
Carlisle JA, Loughton BG (1979) Adipokinetic hormone inhibits protein synthesis in Locusta. Nature 282:420–421
Chipens G (1983) Principles of active site formation in peptides and proteins. In: Peptides, Structure and function (V.J. Hruby and D.H. Rich, eds.) 865–868
Chou PY, Fasman GD (1978) Empirical predictions of protein conformation. Ann Rev Biochem 47:251–276
Christensen M, Carlsen J, Josefsson L (1978) Structure-function studies on red pigment-concentrating hormone; the significance of the terminal residues. Hoppe-Seyler's Z Physiol Chem 359:813–818
Cohen SA, Tarvin TL, Bidlingmeyer BA (1984) Analysis of amino acids using precolumn derivatization with phenylyisothiocyanate. American Laboratory 16:48–59
Ephrussi BE, Beadle GW (1936) Transplantation technique, Drosophila. Amer Nat 70:218–225
Ford MM, Hayes TK, Keeley LL (1988) Structure-activity relationships for insect hypertrehalosemic hormone: The importance of side chains and termini. In: Peptides. Chemistry and biology (G.M. Marshall, ed.) 653–655
Fernlund P, Josefsson L (1972) Crustacean color-change hormone: amino acid sequence and chemical synthesis. Science 177:173–175
Gäde G, Hilbich C, Beyreuther K, Rinehart KL (1988) Sequence analyses of two neuropeptides of the AKH/RPCH-family from the lubber grasshopper, Romalea microptera. Peptides 9:681–688
Gäde G (1986) Relative hypertrehalosaemic activities of naturally occurring neuropeptides from the AKH/RPCH family. Z Naturforsch 41c:315–320
Gäde G, Rinehart KL (1986) Amino acid sequence of a hypertrehalosemic neuropeptide from the corpus cardiacum of the cockroach, Nauphoeta cinerea. Biochem Biophys Res Comm 141:774–781
Gäde G, Rinehart KL (1987a) Primary structure of the hypertrehalosaemic factor II from the corpus cardiacum of the Indian stick insect, Carausius morosus, determined by fast atom mass spectrometry. Biol Chem Hoppe-Seyler 368:67–75
Gäde G, Rinchart KL (1987b) Primary sequence analysis by fast atom bombardment mass spectrometry of a peptide with adipokinetic activity from the corpora cardiaca of the cricket Gryllus bimaculatus. Biochem Biophys Res Comm 149:908–1014
Gäde G, Goldsworthy GJ, Schaffer MH, Cook JC, Rinehart KL (1986) Sequence analyses of adipokinetic hormones II from corpora cardiaca of Schistocerca nitans, Schistocerca gregaria, and Locusta migratoria by fast atom bombardment mass spectrometry. Biochem Biophys Res Comm 134:723–730
Goldsworthy GJ, Mallison K, Wheeler CH, Gäde G (1986) Relative adipokinetic activities of members of the adipokinetic hormone/red pigment concentrating hormone family. J Insect Physiol 32:433–438
Hayes TK, Keeley LL, Knight DW (1986) Insect hypertrehalosemic hormone: Isolation and primary structure from Blaberus discoidalis cockroaches. Biochem Biophys Res Comm 140:674–678
Hayes TK, Keeley LL (1985) Properties of an in vitro bioassay for hypertrehalosemic hormone of Blaberus discoidalis cockroaches. Gen Comp Endocrinol 57:246–256
Holman GM, Wright MS, Nachman RJ (1988) Insect neuropeptides: Coming of age. ISI Atlas of Science: Animal and Plant Sciences 129–136
Hruby VJ (1984) Design of peptide superagonists and antagonists, conformational and dynamic considerations. In: Conformationally directed drug design. (Vida J.A. and Gorden M., eds.) American Chemical Society, Washongton, D.C. 9–27
Hudson D, Kain D, Ng D (1986) High yielding fully automatic synthesis of cecropin A amide and analogues. Peptide chemistry, (ed. Kiso, Y.), Protein Research Found., Osaka
Jaffe H, Raina AK, Riley CT, Fraser BA, Holman GM, Wagner RM, Ridgway RL, Hayes DK (1986) Isolation and primary structure of a peptide from the corpora cardiaca of Heliothis zea with adipokinetic activity. Biochem Biophys Res Comm 135:622–628
Jaffe H, Raina AK, Riley CT, Fraser BA, Bird TG, Tseng CM, Zhang YS, Hayes DK (1988a) Isolation and primary structure of a neuropeptide hormone from Heliothis zea with hypertrehalosemic and adipokinetic activities. Biochem Biophys Res Comm 155:344–350
Jaffe H, Raina AK, Fraser BA, Keim P, Rao KR, Zhang YS, Lancaster JL, Hayes DK (1988b) Isolation of two neuroptides in the AKH/RPCH-family from horseflies (Diptera). Biochem Biophys Res Comm 151:656–663
Mayer RJ, Candy DJ (1969) Control of haemolymph lipid concentration during locust flight: adipokinetic hormone from the corpora cardiaca. J Insect Physiol 15:611–620
Mordue W, Stone JV (1976) Comparison of the biological activities of an insect and a crustacean neurohormone that are structurally similar. Nature 264:287–289
Orr GL, Gole JWD, Jahagirdar AP, Downer RGH, Steele JE (1985) Cyclic AMP does not mediate the action of synthetic hypertrehalosemic peptides from the corpus cardiacum of Periplaneta americana. Insect Biochem 15:703–709
Raina AK, Gäde G (1988) Insect peptide nomenclature. Insect Biochem 18:785–787
Robinson NL, Goldsworthy GJ (1974) The effects of locust adipokinetic hormone on flight muscle metabolism in vivo and in vitro. J Comp Physiol 89:369–377
Roe JH (1955) The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem 212:335–343
Rose GD, Gierasch LM, Smith JA (1985) Turns in peptides and proteins. Advances in Protein Chemistry 37:1–108
Sawyer TK, Cody WL, Knittle JJ, Hruby VJ, Hadley ME, Hirsch MD, O'Donohue TL (1983) Design of conformationally-restricted cyclic a-melanotropins: comparison of melanocytestimulating and behavioral activities. In: Peptides. Structure and function (V.J. Hruby and D.H. Rich, eds.) 323–331
Scarborough RM, Jamieson GC, Kalish F, Kramer SJ, McEnroe GA, Miller CA, Schooley DA (1984) Isolation and primary structure of two peptides with cardioacceleratory and hyperglycemic activity from the corpora cardiaca of Periplaneta americana. Proc Natl Acad Sci U.S.A. 81:5575–5579
Siegert KJ, Morgan P, Mordue W (1985) Primary structures of locust adipokinetic hormones II. Hoppe-Seyler's Z Physiol Chem 366:723–727
Steele JE (1961) Occurrence of a hyperglycemic-factor in the corpus cardiacum of an insect. Nature 192:680–681
Stone JV, Mordue W, Batley KE, Morris HR (1976) Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilization during flight. Nature 263:207–211
Stone JV, Mordue W, Broomfield CT, Hardy PM (1978) Structure-activity relationships for the lipid-mobilising action of locust adipokinetic hormone. Synthesis and activity of a series of hormone analogues. Eur J Biochem 89:195–202
Veber DF (1981) Design of a highly active cyclic hexapeptide analog of somatostatin. In: Peptides, synthesis, structure and function (D.H. Rich and E. Gross, eds.) 685–694
Weeda E (1981) Hormonal regulation of proline synthesis and glucose release in the fat body of the Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol 27:411–417
Ziegler R, Eckart K, Schwarz H, Keller R (1985) Amino acid sequence of Manduca sexta adipokinetic hormone elucidated by combined fast atom bombardment (FAB)/tandem mass spectrometry. Biochem Biophys Res Comm 133:337–342
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hayes, T.K., Keeley, L.L. Structure-activity relationships on hyperglycemia by representatives of the adipokinetic/hyperglycemic hormone family in Blaberus discoidalis cockroaches. J Comp Physiol B 160, 187–194 (1990). https://doi.org/10.1007/BF00300952
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00300952