Abstract
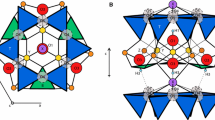
Tourmaline has two different [6]-coordinated sites, the Y site and the Z site. Vacancies were reported from both sites. Based on high-quality chemical and single-crystal structural data it usually needs increasing proportions of short-range order configurations Na(Al2□)Al6(BO3)3[Si6O18]V(OH)3W(OH) or Na(Al2□)Al6(BO3)3[Si6O18]V(OH)3WF in order to produce Y-site vacancies (with □ being the symbol for a vacant site). Less commonly, the short-range configuration Ca(Al2□)Al6(BO3)3[Si5T3+O18]V(OH)3W(OH) could occur in Al-rich tourmalines with a Si deficiency, where T3+ = B, Al. Therefore, tourmalines enriched in cations with charge 2 + (Fe2+, Mn2+, Mg) contain only insignificant Y-site vacancies. Aluminum-rich tourmalines with ≥ 7.0 apfu Altotal that usually contain ≥ 0.2 apfu Li may have significant vacancies at the Y site. However, no more than 12% vacancies (0.36 pfu) at the Y site can be observed in such samples. If no chemical data for Li is available it is proposed to calculate the Li content in such colourless or coloured tourmalines (elbaite, fluor-elbaite, fluor-liddicoatite, rossmanite) for Y = 2.8 apfu or for Y + Z + T = 14.8 apfu, because this calculation should give more accurate results than calculating the Li content as the difference to 3.0 apfu at the Y site. For Fe2+-rich and Mg-bearing tourmalines from the schorl-dravite series with MgO > 1.0 wt% (and only minor amounts of Fe3+, Cr3+ and V3+) the structural formula can still be calculated for Y + Z + T = 15 apfu, because such tourmalines do not appear to contain significant Y-site vacancies. It can further be concluded that the Z site could be only ≤ 1% vacant and therefore such vacancies would be insignificant even in Al-rich tourmaline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The generalized formula of tourmaline-supergroup minerals can be written as XY3Z6(T6O18)(BO3)3V3W, as proposed by Henry et al. (2011). These authors and Hawthorne (1996, 2002) suggest occupancies by the following most common cations:
-
IXX = Na+, Ca2+, □
-
VIY = Fe2+, Mg2+, Al3+, Li+, Mn2+, Fe3+, Cr3+, V3+
-
VIZ = Al3+, Mg2+, Fe3+, Cr3+, V3+
-
IVT = Si4+, Al3+, B3+
-
IIIB = B3+
-
IIIV = OH–, O2–
-
IIIW = OH–, F–, O2–
Some of these cations can be present simultaneously on two and even three structural sites, reflecting order–disorder phenomena, mainly between the octahedral Y- and Z-site occupants (Ertl et al. 2003 and references therein). The tourmaline supergroup currently comprises more than 40 valid mineral species accepted by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA). They represent hydroxyl-, fluor- and oxy-species of X-site vacant, alkali, and calcic tourmalines with typical octahedral occupants as listed above (Henry et al. 2011). Crystal-chemical relations in the tourmaline supergroup and the crystal chemistry of tourmaline supergroup minerals have been investigated by many authors in the last 50 years (e.g., Donnay and Barton 1972; Povondra and Čech 1976; Deer et al. 1986; Hawthorne et al. 1993; Hawthorne 1996, 2002, 2016; Henry and Dutrow 1996; Ertl et al. 2002, 2012a, 2018; Bosi and Lucchesi 2004, 2007; Bosi et al. 2004, 2013, 2017; Hughes et al. 2004; Ertl and Tillmanns 2012; Ertl and Bačík 2020; Bačík and Fridrichová 2021). Tourmaline can also be a petrologic recorder of its geologic history as was shown by Van Hinsberg et al. (2011).
While vacancies at the [9]-coordinated X site are commonly well accepted because in some cases they may even dominate this site (foitite, oxy-foitite, magnesiofoitite, rossmanite, alumino-oxy-rossmanite), vacancies at the [6]-coordinated sites usually have generally not been characterized and described as well. Based on published high-quality data, the present work considers whether, why and when vacancies occur in the [6]-coordinated sites and in which tourmalines they would be expected. Any effects of such possible vacancies are also discussed.
Previous work
The tourmaline crystal structure contains two different [6]-coordinated sites. Since more than 50 years, the site with the larger (distorted) octahedra is named the Y site and the site with the smaller (distorted) octahedra is named the Z site. Articles from the 1970ies and 1980ies, in which small amounts of Y-site vacancies, up to 0.16 per formula unit (pfu), have been cited, were summarized in Table 5 of Foit (1989). Many structural refinements in combination with chemical analyses indicate in later works that a minor Y-site vacancy could possibly exist (e.g., Hawthorne et al. 1993; Taylor et al. 1995; Ertl et al. 1997, 2003, 2006, 2009, 2010a, b).
Tourmaline samples of Mn-bearing fluor-elbaite, which were characterised chemically (including B2O3, Li2O, and H2O analysis) and by single crystal structure, show up to 0.21 pfu Y-site vacancies (Ertl et al. 2010a, b). Smaller Y-site vacancies up to ~ 0.10 pfu have been reported in in YAl-bearing schorl samples, which have also been fully characterised (Ertl et al. 2016). Higher Y-site vacancies (up to 0.19 pfu) have been reported from very Al-rich tourmalines with a dominant alumino-oxy-rossmanite component, i.e. with a vacancy-dominant X-site and with some tetrahedrally coordinated Al (Ertl et al. 2022). Even higher Y-site vacancies of 0.24 pfu were described from an Al-rich tourmaline, also with a vacancy-dominant X site, referred to as rossmanite (Ertl et al. 2009). Similar Y-site vacancies (up to 0.25 pfu) have been reported from B-rich olenite, also an Al-rich tourmaline (Ertl and Brandstätter 1998; Ertl et al. 2007, 2018). Interestingly, a Fe-rich and Mg-bearing tourmaline was described with 0.30 Y-site vacancies (Filip et al. 2012). These authors described a rather unusual tourmaline, which, in addition to Fe2+ and some Al at the Y-site, contains a relatively large amount of Fe3+. The samples with the highest reported Y-site vacancies are Al-rich tourmalines: a Mn- and Fe-bearing fluor-elbaite with 0.35 pfu vacancies (Grew et al. 2018) and a Fe-, Mn-, and Li-bearing olenite with 0.36 pfu vacancies (Ertl et al. 2012b). A natural Al-rich foititic tourmaline with ~ 0.35 pfu Y-site vacancies was also described (Wodara and Schreyer 2001). However, this sample lacks chemical analyses of B and H and no single-crystal structure refinement was performed.
In addition, from synthetic Al-rich tourmalines Y-site vacancies were described. Tourmalines, enriched in Al and Cu, showed up to 0.12 pfu Y-site vacancies (Ertl et al. 2015). Very Al- and B-rich tourmaline (olenite), a columnar crystal, was reported with ~ 0.17 pfu Y-site vacancies, which were determined by single-crystal structure refinement (Kutzschbach et al. 2016). Very Al- and B-rich olenite, synthesized by Wodara and Schreyer (1997), was described in an updated structural formula with 0.30 pfu Y-site vacancies (Ertl and Brandstätter 1998). The authors finally described X-site vacant and Al- and B-rich tourmaline with a structural formula containing ~ 0.29 pfu Y-site vacancies (Wodara and Schreyer 2001). Unfortunately, no single-crystal structure refinement was performed on the last two cited samples.
There are almost no publications describing Z-site vacancies. In an Al- and B-rich tourmaline (olenite), 0.08 pfu Z-site vacancies (for 6 Z sites) were reported, based on chemical and structural data (Hughes et al. 2000). These authors used a method of optimizing the site occupancies of cation sites in minerals with multiply occupied cation sites (Wright et al. 2000).
An extensive theoretical work by using bond-valence theory to explore the local arrangements involving vacancies at the Y and Z sites in the tourmaline structure was published by Bosi (2010). The results of this work show that arrangements involving tetrahedrally coordinated R3+-cations and octahedrally coordinated YR2+- and ZR2+-cations around the oxygen atoms O8, O7 and O6 can be ruled out, together with arrangements involving vacancies and YLi1+. Based on bond-valence calculations Bosi (2010) concluded that vacancies are more favoured to occur at the Y site rather than at the Z site, in tandem with OH− at the V and W sites, R3+ at the octahedral sites and Si4+ at the T site.
Discussion
Usually, the best way to detect Y-site vacancies is when a tourmaline is fully characterised, including chemical analyses of the light elements B, Li, and H. If the tourmaline formula is normalized to 15 atoms per formula unit (apfu) for the sum of the occupants at the Y + Z + T sites, or for Y + Z + T + B = 18 apfu, it is clear that possible Y-site occupancies cannot be found. Tourmalines, which contain significant amounts of Mg, usually contain almost no Li and almost no tetrahedrally coordinated B. For such tourmalines, it is therefore not necessary to analyse B in most cases, since they usually contain exactly 3 apfu B. Since essentially Al-rich tourmaline samples with significant Y-site vacancies have been reported in the literature, only such samples were used for plotting correlations. Only the best-characterised tourmalines were used, most samples of which were analysed both chemically (including the light elements) and by single crystal X-ray diffraction. Some of the very Al-rich samples contain significantly less Si than 6 apfu. Hence, two different correlations were plotted, one with ~ 6 apfu Si (Fig. 1) and another with ~ 5 apfu Si (Fig. 2). Since the V site is filled with OH in most tourmalines and the W-site has a mixed occupancy of OH, F and O, it was of interest how the W-site charge interacts with the Y-site vacancies. If the W site has an occupancy of OH + F = 1, the W-site charge is -1. An oxy-component produces a lower W-site charge. If the W site were occupied only by oxygen, the W-site charge would be -2. Both figures show that the observed Y-site vacancy increases, when the oxy-component in tourmaline gets lower. This means that Al-rich tourmalines, where the W site is completely filled by OH + F, can have the highest Y-site vacancies. But when does the oxy-component increase in such samples? It increases when a tourmaline contains components of the endmembers darrellhenryite, dutrowite, lucchesiite, magnesio-lucchesiite, oxy-foitite, oxy-schorl or princivalleite, (not included here are V3+-, Cr3+-, and Fe3+-rich tourmalines). This means that the oxy-component at the W site usually increases, when such a tourmaline contains increasing amounts of (Fe2+, Mn2+, Mg2+, Li1+) and/or increasing X-site vacancies. Hence, the following substitutions can theoretically occur in Al-rich tourmalines to produce Y-site vacancies:
-
1.
2Y2+ + WO = > YAl3+ + Y□ + W(OH,F)
-
2.
XCa2+ + 3Y2+ + WO = > XNa1+ + 2YAl3+ + Y□ + W(OH,F)
-
3.
X□ + Y2+ + WO = > XNa1+ + Y□ + W(OH,F)
-
4.
Y2+ + T3+ + WO = > Y□ + TSi4+ + W(OH,F)
-
5.
X□ + YAl3+ + T3+ + WO = > XNa1+ + Y□ + TSi4+ + W(OH,F)
-
6.
YLi + WO = > Y□ + W(OH,F)
-
7.
XNa1+ + 3Y2+ + TSi4+ + WO = > XCa2+ + 2YAl3+ + Y□ + T3+ + W(OH)
-
8.
XCa2+ + 2YLi1+ + YAl3+ + WF = > XNa1+ + 2YAl3+ + Y□ + W(OH)
-
Y2+: Fe2+, Mn2+, Mg2+
-
T3+: Al3+, B3+
-
Therefore, Mg-poor tourmalines with stoichiometric Si contents can have increasing Y-site vacancies when they are richer in Al and when they contain less (Fe2+, Mn2+ and Mg2+) cations, respectively. If such tourmalines exhibit low X-site vacancies, they will only have small amounts of [4]Al and/or [4]B. Such compositions will come close to a W-site occupancy of OH + F = 1. Regardless of whether tourmaline has a Si deficiency or not, it usually needs increasing proportions or short-range order configurations of Na(Al2□)Al6(BO3)3[Si6O18]V(OH)3 with OH or F at the W site in order to produce Y-site vacancies. A similar short-range order configuration (with OH at the W site) for Al- and Mn2+-rich tourmaline was proposed by Ertl et al. (2003). Later, for the elbaite-schorl series, short-range orders with (OH/F) at the W site were assumed (Ertl et al. 2010a). And indeed, a tourmaline was recently described that consists of 39% fluor-elbaite, 34% of the component NaY(Al2□)ZAl6(BO3)3[Si6O18]V(OH)3WF, 16% fluor-tsilaisite and 11% fluor-schorl (Grew et al. 2018).
It is likely that these proposed substitutions depend not only on the chemistry of the whole rock, but also on the pressure and temperature conditions during the crystallization of tourmaline. Therefore, it would be helpful to have a larger number of tourmaline analyses of each locality. In general, complete published data of Al-rich tourmaline from a locality, including light elements and structural data, are not very common. Therefore, one is limited to check the proposed substitutions. There is, however, one locality from which such tourmalines have been extensively investigated. Therefore, the correlation between Al content at the Y site and Y-site vacancies was checked by using the Al-rich tourmalines of the Koralpe, Austria (Fig. 3). A strong positive correlation between Al content at the Y site and Y-site vacancies was observed. While the amount of Al3+ increases, the amount of Fe2+, Mg2+ and Si4+ decreases. Simultaneously, the amounts of cations with the charge 3 + (B, Al) increase at the tetrahedral site (Table 1 in Kalt et al. 2001). Therefore, it can be concluded that not only substitution (1) but also substitution (7) occurs. The latter substitution would require a short-range order configuration of Ca(Al2□)Al6(BO3)3[Si5T3+O18]V(OH)3W(OH), where T3+ = B, Al. This would be the only proposed substitution with a short-range order configuration, where the T site is not completely filled with Si4+. However, a calculation using bond-valence theory shows that this arrangement also appears to be stable.
Correlation between Al content at the Y site and Y-site vacancies. All samples (fluor-liddicoatite – elbaite series with.ZAl6.0) are from Anjanabonoina, Madagascar (Ertl et al. 2006)
In tourmalines of the fluor-liddicoatite – elbaite series from Anjanabonoina, Madagascar, investigated by Ertl et al. (2006), the vacancies increase also with increasing Al content at the Y site as can be seen in Fig. 4. Because the amounts of Fe2+ and Mn2+ are extremely small in all tourmalines examined, it seems that the substitution (8) is mainly responsible for the Y-site vacancies. This is the only proposed substitution in which the W site is not occupied by oxygen but by fluorine. Interestingly, in substitution (8), an increasing Ca content does not lead to an increasing content of tetrahedrally coordinated B and Al, as in substitution (7). It can only be assumed that the tourmalines from Anjanabonoina, Madagascar crystallized at different temperatures and pressures than the tourmalines from the Koralpe, Austria. Anyway, such vacancies are unlikely to result from incorrect Li2O secondary ion mass spectrometry (SIMS) values, since the Li content is relatively low in many samples examined. The Al-richest sample from the study Ertl et al. (2006), which contains 0.89 apfu Li, yields 0.27 Y-site vacancies in the structural formula. The even more Al-enriched tourmalines (olenite samples) are actually lower in Li and therefore, assuming no Y-site vacancies, the calculated Li2O content would be much too high (> 30%) compared to the measured Li2O content for these samples. Furthermore, Al cannot fill these vacancies since Al has significantly more electrons than Li and therefore the calculated electron occupancy would be much too high compared to the observed electron occupancy at the Y site.
There are also other occupants than Al at the Y site that have a 3 + charge, such as Fe3+, Mn3+, Cr3+ and V3+. Apart from a sample with a relatively high content of Fe3+, described by Filip et al. (2012) it is not yet clear whether tourmalines enriched with these cations could also have Y-site vacancies. In order to find and understand further relationships in detail, however, more detailed investigations must be carried out.
Conclusions
Based on high-quality available data, it can be concluded that significant Y-site vacancies can exist at least in Al-rich tourmalines. In particular, tourmalines with ≥ 7.0 apfu Altotal with usually ≥ 0.2 apfu Li may contain > 0.1 pfu vacancies at the Y site. However, no more than 12% vacancies (0.36 pfu) at the Y site appear to occur. It can be concluded that the calculation of the Li content by completely filling the Y site with Li when no chemical analysis is available is problematic for such Al-rich tourmalines from lithium pegmatites. The average Y-site vacancies in completely characterised Al-rich and Li-bearing (colourless – coloured) tourmalines (elbaite, fluor-elbaite, fluor-liddicoatite, rossmanite) is 0.185(7) (20 samples; Dyar et al. 1998; Ertl et al. 2006, 2009, 2010a, 2012b; Grew et al. 2018). Therefore, it is proposed to calculate the Li content in such tourmalines, if no chemical data is available, for Y = 2.8 apfu or Y + Z + T = 14.8 apfu or for Y + Z + T + B = 17.8 apfu. This calculation should give more accurate results than calculating the Li content as the difference to 3.0 apfu at the Y site.
Chemical data of Novák et al. (2004) showed that for (Fe,Mg)-rich, (Ca,Li,F)-poor tourmalines a W-site occupancy with [(OH,F)0.5O0.5] is more probable than with (OH,F)1.0, particularly in the tourmaline with a X-site vacancy > 0.3 pfu. Based on the trend seen in Fig. 1, no significant Y-site vacancies are expected in such tourmalines. Further investigations on Fe2+-rich and Mg-rich tourmalines have shown that samples with MgO > 1.0 wt% typically contain < 0.10 pfu Y-site vacancies (Bloodaxe et al. 1999; Ertl et al. 2012b). It cannot be ruled out that very small, calculated Y-site vacancies could be the result of random analytical errors propagating from measurements of oxides including light elements. Metamorphic tourmalines have > 0.2 apfu Mg and have very small amounts of Li, and thus, Li is typically an insignificant constituent in metamorphic tourmaline (Henry and Dutrow 1996). Therefore, no significant Y-site vacancies are expected in metamorphic tourmaline either. It can be concluded that for Fe2+-rich and Mg-bearing tourmalines with MgO > 1.0 wt% (and only minor amounts of Fe3+, Cr3+ and V3+) the structural formula can be calculated for Y + Z + T = 15 apfu, or for Y + Z + T + B = 18 apfu, because such tourmalines contain no significant Y-site vacancies.
It can be assumed that possible vacancies at the Z site could be ≤ 0.05 apfu (for 6 Z sites), which corresponds to ≤ 1% vacancies, and thus would be insignificant. The highest chance of detecting Z-site vacancies in tourmalines is in samples with a very large amount of [6]-coordinated cations with a 3 + charge, e.g., in Al-rich tourmalines. Further investigations will be necessary to obtain more high-quality data to be able to definitively prove Z-site vacancies. These conclusions are consistent with the theoretical approach of Bosi (2010) who, using bond-valence theory, predicted that vacancies occur at the Y site rather than the Z site.
References
Bačík P, Fridrichová J (2021) Cation partitioning among crystallographic sites based on bond-length constraints in tourmaline-supergroup minerals. Am Mineral 106:851–861
Bloodaxe ES, Hughes JM, Dyar MD, Grew ES, Guidotti CV (1999) Linking structure and chemistry in the Schorl-Dravite series. Am Mineral 84:922–928
Bosi F (2010) Octahedrally coordinated vacancies in tourmaline: A theoretical approach. Min Mag 74:1037–1044
Bosi F, Lucchesi S (2004) Crystal chemistry of the schorl-dravite series. Eur J Mineral 16:335–344
Bosi F, Lucchesi S (2007) Crystal chemical relationships in the tourmaline group: structural constraints on chemical variability. Am Mineral 92:1054–1063
Bosi F, Cámara F, Ciriotti ME, Hålenius U, Reznitskii L, Stagno V (2017) Crystal chemical relations and classification problems of tourmalines belonging to the oxy-schorl–oxy-dravite–bosiite–povondraite series. Eur J Mineral 29:445–455
Bosi F, Lucchesi S, Reznitskii L (2004) Crystal chemistry of the dravitechromdravite series. Eur J Mineral 16:345–352
Bosi F, Skogby H, Hålenius U, Reznitskii L (2013) Crystallographic and spectroscopic characterization of Fe-bearing chromo-alumino-povondraite and its relations with oxy-chromium-dravite and oxy-dravite. Am Mineral 98:1557–1564
Deer WA, Howie RA, Zussman J (1986) Rock-forming Minerals. Disilicates and Ring Silicates 1B(2):608. Longman
Donnay G, Barton R Jr (1972) Refinement of the crystal structure of elbaite and the mechanism of tourmaline solid solution. Tschermaks Mineral Petrogr Mitt 18:273–286
Dyar MD, Taylor ME, Lutz TM, Francis CA, Guidotti CV, Wise M (1998) Inclusive chemical characterization of tourmaline: Mössbauer study of Fe valence and site occupancy. Am Mineral 83:848–864
Ertl A, Brandstätter F (1998) Olenit mit Borüberschuß aus einem Metapegmatit östlich der Stoffhütte, Koralpe, Steiermark, Österreich. Mitteilungen Der Abteilung Für Mineralogie Am Landesmuseum Joanneum 62(63):3–21
Ertl A, Bačík P (2020) Considerations about Bi and Pb in the crystal structure of Cu-bearing tourmaline. Minerals 10:706
Ertl A, Giester G, Ludwig T, Meyer H-P, Rossman GR (2012a) Synthetic B-rich olenite: Correlations of single-crystal structural data. Am Mineral 97:1591–1597
Ertl A, Henry DJ, Tillmanns E (2018) Tetrahedral substitutions in tourmaline: A review. Eur J Mineral 30:465–470
Ertl A, Hughes JM, Pertlik F, Foit FF Jr, Wright SE, Brandstätter F, Marler B (2002) Polyhedron distortions in tourmaline. Can Mineral 40:153–162
Ertl A, Hughes JM, Prowatke S, Ludwig T, Brandstätter F, Körner W, Dyar MD (2007) Tetrahedrally-coordinated boron in Li-bearing olenite from “mushroom” tourmaline from Momeik, Myanmar. Can Mineral 45:891–899
Ertl A, Hughes JM, Prowatke S, Ludwig T, Lengauer CL, Meyer HP, Giester G, Kolitsch U, Prayer A (2022) Alumino-oxy-rossmanite from pegmatites in Variscan metamorphic rocks from Eibenstein an der Thaya, Lower Austria, Austria: A new tourmaline that represents the most Al-rich end-member composition. Am Mineral 107:157–166
Ertl A, Hughes JM, Prowatke S, Ludwig T, Prasad PS, Brandstätter F, Körner W, Schuster R, Pertlik F, Marschall H (2006) Tetrahedrally-coordinated boron in tourmalines from the liddicoatite-elbaite series from Madagascar: Structure, chemistry, and infrared spectroscopic studies. Am Mineral 91:1847–1856
Ertl A, Hughes JM, Prowatke S, Rossman GR, London D, Fritz EA (2003) Mn-rich tourmaline from Austria: structure, chemistry, optical spectra, and relations to synthetic solid solutions. Am Mineral 88:1369–1376
Ertl A, Kolitsch U, Dyar MD, Meyer H-P, Rossman GR, Henry DJ, Prem M, Ludwig T, Nasdala L, Lengauer CL, Tillmanns E, Niedermayr G (2016) Fluor-schorl, a new member of the tourmaline supergroup, and new data on schorl from the cotype localities. Eur J Mineral 28:163–177
Ertl A, Kolitsch U, Meyer H-P, Ludwig T, Lengauer CL, Nasdala L, Tillmanns E (2009) Substitution mechanism in tourmalines of the “fluor-elbaite”-rossmanite series from Wolkenburg, Saxony, Germany. N Jb Miner Abh 186:51–61
Ertl A, Mali H, Schuster R, Körner W, Hughes JM, Brandstätter F, Tillmanns E (2010b) Li-bearing, disordered Mg-rich tourmalines from the pegmatite-marble contact from the Austroalpine basement units (Styria, Austria). Miner Petrol 99:89–104
Ertl A, Rossman GR, Hughes JM, London D, Wang Y, O’Leary JA, Dyar MD, Prowatke S, Ludwig T, Tillmanns E (2010a) Tourmaline of the elbaite-schorl series from the Himalaya Mine, Mesa Grande, California, USA.: A detailed investigation. Am Mineral 95:24–40
Ertl A, Pertlik F, Bernhardt H-J (1997) Investigations on olenite with excess boron from the Koralpe, Styria, Austria. Österreichische Akademie der Wissenschaften, Mathematisch-naturwissenschaftliche Klasse. Abteilung I, Anzeiger 134:3–10
Ertl A, Schuster R, Hughes JM, Ludwig T, Meyer H-P, Finger F, Dyar MD, Ruschel K, Rossman GR, Klötzli U, Brandstätter F, Lengauer CL, Tillmanns E (2012b) Li-bearing tourmalines in Variscan pegmatites from the Moldanubian nappes, Lower Austria. Eur J Mineral 24:695–715
Ertl A, Tillmanns E (2012) The [9]-coordinated X site in the crystal structure of tourmaline-group minerals. Z Krist 227:456–459
Ertl A, Vereshchagin OS, Giester G, Tillmanns E, Meyer HP, Ludwig T, Rozhdestvenskaya IV, Frank-Kamenetskaya OV (2015) Structural and chemical investigation of a zoned synthetic Cu-rich tourmaline. Can Mineral 53:209–220
Filip J, Bosi F, Novák M, Skogby H, Tučel J, Čuda J, Wildner M (2012) Iron redox reactions in the tourmaline structure: High-temperature treatment of Fe3+-rich schorl. Geochim Cosmochim Acta 86:239–256
Foit FF Jr (1989) Crystal chemistry of alkali-deficient schorl and tourmaline structural relationships. Am Mineral 74:422–431
Grew ES, Bosi F, Ros L, Kristiansson P, Gunter ME, Hålenius U, Trumbull RB, Yates MG (2018) Fluor-elbaite, lepidolite and Ta–Nb oxides from a pegmatite of the 3000 Ma Sinceni Pluton, Swaziland: evidence for lithium–cesium–tantalum (LCT) pegmatites in the Mesoarchean. Eur J Mineral 30:205–218
Hawthorne FC (1996) Structural mechanisms for light-element variations in tourmaline. Can Mineral 34:123–132
Hawthorne FC (2002) Bond-valence constraints on the chemical composition of tourmaline. Can Mineral 40:789–797
Hawthorne FC (2016) Short-range atomic arrangements in minerals. I: The minerals of the amphibole, tourmaline and pyroxene supergroups. Eur J Mineral 28:513–536
Hawthorne FC, MacDonald DJ, Burns PC (1993) Reassignment of cation site-occupancies in tourmaline: Al-Mg disorder in the crystal structure of dravite. Am Mineral 78:265–270
Henry DJ, Dutrow BL (1996) Metamorphic tourmaline and its petrologic applications. Rev Mineral Geochem 33:503–557
Henry DJ, Novák M, Hawthorne FC, Ertl A, Dutrow BL, Uher P, Pezzotta F (2011) Nomenclature of the tourmaline-supergroup minerals. Am Mineral 96:895–913
Hughes JM, Ertl A, Dyar MD, Grew E, Shearer CK, Yates MG, Giudotti CV (2000) Tetrahedrally coordinated boron in a tourmaline: Boron-rich olenite from Stoffhütte, Koralpe, Austria. Can Mineral 38:861–868
Hughes JM, Ertl A, Dyar MD, Grew ES, Wiedenbeck M, Brandstätter F (2004) Structural and chemical response to varying [4]B content in zoned Fe-bearing olenite from Koralpe, Austria. Am Mineral 89:447–454
Kalt A, Schreyer W, Ludwig T, Prowatke S, Bernhardt H-J, Ertl A (2001) Complete solid solution between magnesian schorl and lithian excess-boron olenite in a pegmatite from Koralpe (eastern Alps, Austria). Eur J Mineral 13:1191–1205
Kutzschbach M, Wunder B, Rhede D, Koch-Müller M, Ertl A, Giester G, Heinrich W, Franz G (2016) Tetrahedral boron in natural and synthetic high-pressure tourmaline: Evidence from Raman spectroscopy, EMPA, and single-crystal XRD. Am Mineral 101:93–104
Novák M, Povondra P, Selway JB (2004) Schorl–oxy-schorl to dravite–oxy-dravite tourmaline from granitic pegmatites; examples from the Moldanubicum, Czech Republic. Eur J Mineral 16:323–333
Povondra P, Čech A (1976) A method for the chemical analysis of tourmaline. Acta Universitatis Carolinae Geologica 209–218
Schreyer W, Wodara U, Marler B, van Aken PA, Seifert F, Robert J-L (2000) Synthetic tourmaline (olenite) with excess boron replacing silicon in the tetrahedral site. I. Synthesis conditions, chemical and spectroscopic evidence. Eur J Mineral 12:529–541
Taylor MC, Cooper MA, Hawthorne FC (1995) Local charge-compensation in hydroxyl-deficient uvite. Can Mineral 33:1215–1221
Van Hinsberg VJ, Henry DJ, Dutrow BL (2011) Tourmaline as a petrologic forensic mineral: A unique recorder of its geologic past. Elements 7:327–332
Wodara U, Schreyer W (1997) Turmaline mit Borüberschuß im System Na2O-Al2O3-B2O3-SiO2-H2O (NABSH). Berichte der Deutschen Mineralogischen Gesellschaft, Beihefte z. Eur J Mineral 9:394
Wodara U, Schreyer W (2001) X-site vacant Al-tourmaline: a new synthetic endmember. Eur J Mineral 13:521–532
Wright SE, Foley JA, Hughes JM (2000) Optimization of site-occupancies in minerals using quadratic programming. Am Mineral 85:524–531
Acknowledgements
Special thanks to Peter Bačík and Beatrice Celata for their constructive reviewer comments as well as to Guest Editor Karen Friese for handling this article. This study was funded in whole by the Austrian Science Fund (FWF) project P 35585. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Funding
Open access funding provided by Austrian Science Fund (FWF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: K. Friese
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ertl, A. Are the [6]-coordinated sites in tourmaline in certain cases partially vacant?. Miner Petrol 117, 201–207 (2023). https://doi.org/10.1007/s00710-023-00815-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-023-00815-4