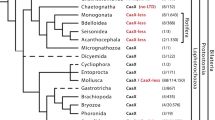
Abstract
We identified 23 and 20 cytoplasmic IF (cIF) genes in the two Branchiostoma belcheri and Branchiostoma lanceolatum cephalochordates, respectively. Combining these results with earlier data on the related Branchiostoma floridae, the following conclusions can be drawn. First, the Branchiostoma N4 protein with a long lamin-like coil 1B segment is the only protostomic-type cIF found so far in any analysed chordate or vertebrate organism. Second, Branchiostoma is the only organism known so far containing both the long protostomic- and the short chordate-prototypes of cIFs. This finding provides so far missing molecular evidence for the phylogenetic transition between the protostomic- and the chordate-type IF sequences at the base of the cephalochordates and vertebrates. Third, this finding also brings some support for another hypothesis, that the long protostomic-type cIF is subjected to evolutionary constraints in order to preclude inappropriate interactions with lamin and that the latter complexes might be prevented by a several heptad-long rod deletion, which released the selective constraints on it and promoted, at least in part, its expansion in nematodes, cephalochordates, and in vertebrates. Finally, here-presented data confirmed our previous results that cephalochordates do not have any vertebrate type III or type IV IF homolog.


Similar content being viewed by others
References
Aebi U, Cohn J, Buhle L, Gerace L (1986) The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323:560–564
Chernyatina AA, Guzenko D, Strelkov SV (2015) Intermediate filament structure: the bottom-up approach. Cur Opin Cell Biol 32:65–72
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36(Web server issue):W465–W469
Döring V, Stick R (1990) Gene structure of nuclear lamin LIII of Xenopus laevis; a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J 9:4073–4081
Ehrlich F, Fischer H, Langbein L, Praetzel-Wunder S, Ebner B, Figlak K, Weissenbacher A, Sipos W, Tschachler E, Eckhart L (2018) Differential evolution of the epidermal keratin cytoskeleton in terrestrial and aquatic mammals. Mol Biol Evol 36(2):328–340
Eldirany SA, Lomakin IB, Ho M, Bunick CG (2021) Recent insight to intermediate filament structure. Cur Opin Cell Biol 68:132–143
Erber A, Riemer D, Bovenschulte M, Weber K (1998) Molecular phylogeny of metazoan intermediate filament proteins. J Mol Evol 47:751–762
Erber A, Riemer D, Hofemeister H, Bovenschulte M, Stick R, Panopoulou G, Lehrach H, Weber K (1999) Characterisation of the Hydra lamin and its gene; a molecular phylogeny of metazoan lamins. J Mol Evol 49:260–271
Fuchs E, Weber K (1994) Intermediate filaments: structure, dynamics, function and disease. Annu Rev Biochem 63:345–382
Geisler F, Coch RA, Richardson C, Goldberg M, Denecke M, Bossinger O, Leube RE (2019) The intestinal intermediate filament network responds to and protects against microbial insults and toxins. Development 146(2):pii: dev169482. https://doi.org/10.1242/dev.169482
Geisler N, Schünemann J, Weber K, Häner M, Aebi U (1998) Assembly and architecture of invertebrate cytoplasmic intermediate filaments reconcile features of vertebrate cytoplasmic and nuclear lamin-type intermediate filaments. J Mol Biol 282:601–617
Herrmann H, Hesse M, Reichenzeller M, Aebi U, Magin TM (2003) Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int Rev Cytol 223:83–175
Herrmann H, Strelkov SV, Burkhard P, Aebi U (2009) Intermediate filament: primary determinants of cell architecture and plasticity. J Clin Invest 119:1772–1783
Hesse M, Franz T, Tamai Y, Taketo MM, Magin TM (2000) Targeted deletion of keratin 18 and 19 leads to trophoblast fragility and early embryonic lethality. EMBO J 19:5060–5070
Hesse M, Zimek A, Weber K, Magin TM (2004) Comprehensive analysis of keratin gene clusters in humans and rodents. Eur J Cell Biol 83:19–26
Ho M, Thompson B, Fisk JN, Nebert DW, Bruford EA, Vasiliou V, Bunick CH (2022) Update of the keratin gene family: evolution, tissue-specific expression patterns, and relevance to clinical disorders. Hum Genomics 16:1. https://doi.org/10.1186/s40246-021-00374-9
Karabinos A (2013) The cephalochordate Branchiostoma genome contains 26 intermediate filament (IF) genes: implication for evolution of chordate IF proteins. Eur J Cell Biol 92(8-9):295–302
Karabinos A (2019) Intermediate filament (IF) proteins IFA-1 and IFB-1 represent a basic heteropolymeric IF cytoskeleton of nematodes: a molecular phylogeny of nematode IFs. Gene 692:44–53
Karabinos A, Riemer D, Erber A, Weber K (1998) Homologues of vertebrate type I, II and III intermediate filament (IF) proteins in an invertebrate; the IF multigene family of the cephalochordate Branchiostoma. FEBS Lett 437:15–18
Karabinos A, Riemer D, Panopoulou G, Lehrach H, Weber K (2000) Characterisation and tissue-specific expression of the two keratin subfamilies of intermediate filament proteins in the cephalochordate Branchiostoma. Eur J Cell Biol 79:1–10
Karabinos A, Schünemann J, Meyer M, Aebi U, Weber K (2003) The single nuclear lamin of Caenorhabditis elegans forms in vitro stable intermediate filaments and paracrystals with a reduced axial periodicity. J Mol Biol 325:241–247
Karabinos A, Zimek A, Weber K (2004) The genome of the early chordate Ciona intestinalis encodes only five cytoplasmic intermediate filament proteins including a single type I and type II keratin and a unique IF-annexin fusion protein. Gene 326:123–129
Karabinos A, Wang J, Wenzel D, Panopoulou G, Lehrach H, Weber K (2001a) Developmentally controlled expression pattern of intermediate filament proteins in the cephalochordate Branchiostoma. Mech Dev 101:283–288
Karabinos A, Schmidt H, Harborth J, Schnabel R, Weber K (2001b) An essential role for four intermediate filament proteins in Caenorhabditis elegans development. Proc Natl Acad Sci USA 98:7863–7868
Karabinos A, Schünemann J (2014) Unusual ultrastructures of the Branchiostoma IF protein C2 containing heptads in the tail. Protoplasma 251(4):985–988. https://doi.org/10.1007/s00709-013-0608-6
Karabinos A, Schünemann J, Parry DAD, Weber K (2002) Tissue-specific co-expression and in vitro heteropolymer formation of the two small Branchiostoma intermediate filament proteins A3 and B2. J Mol Biol 316:127–137
Karabinos A, Schünemann J, Parry DAD (2012) A rod domain sequence in segment 1B triggers dimerisation of the two small Branchiostoma IF proteins B2 and A3. Eur J Cell Biol 91:800–808
Karabinos A, Schünemann J, Parry DAD (2019) Promiscuous dimerization between the Caenorhabditis elegans IF proteins and a hypothesis to explain how multiple IFs persist over evolutionary time. J Mol Evol 87(7-8):221–230
Kimura Y, Nikaido M (2021) Conserved keratin gene clusters in ancient fish: an evolutionary seed for terrestrial adaptation. Genomics 113:1120–1128
Kollmar M (2015) Polyphyly of nuclear lamin genes indicates an early eukaryotic origin of the metazoan-type intermediate filament proteins. Sci Rep 5:10652. https://doi.org/10.1038/srep10652
Maggi L, Mavroidis M, Psarras S, Capetanaki Y, Lattanzi G (2021) Skeletal and cardiac muscle disorders caused by mutations in genes encoding intermediate filament proteins. Int J Mol Sci 22:4256. https://doi.org/10.3390/ijms22084256
Parry DAD (2021) Structure of the b-keratin filaments and keratin intermediate filaments in the epidermal appendages of birds and reptiles (Sauropsids). Genes 12:591. https://doi.org/10.3390/genes12040591
Parry DAD, Steinert PM (1995) Intermediate filament structure. Springer, New York
Parry DAD, Winter DJ (2021) Keratin intermediate filament chains in tuat,ara (Sphenodon punctatus): a comparison of tuatara and human sequences. J Str Biol 213(1):107706. https://doi.org/10.1016/j.jsb.2021.107706
Peter A, Stick R (2015) Evolutionary aspects in intermediate filament proteins. Curr Opin Cell Biol 32:48–55
Peter A, Stick R (2023) CaaX-less lamins: Lophotrochozoa provide a glance at the playground of evolution. Protoplasma 260(3):741–756
Putnam HN, Butts T, Ferrier DEK, Furlong RF, Hellsten U, Kawashima T, Robinson-Rachevi M, Shoguchi E, Terry A, Yu JK, Benito-Gutierrez E, Dubchak I, Garcia-Fernandez J, Gibson-Brown JJ, Grigoriev IV, Horton AC, de Jong PJ, Jurka J, Kapitonov VV et al (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453:1064–1071
Razafsky D, Hodzic D (2009) Bringing KASH underthe SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol 186(4):461–472
Riemer D, Karabinos A, Weber K (1998) Analysis of eight cDNAs and six genes of intermediate filament (IF) proteins in the cephalochordate Branchiostoma reveals differences in the IF multigene families of lower chordates and the vertebrates. Gene 211:361–373
Vandebergh W, Bossuyt F (2012) Radiation and functional diversification of alpha keratins during early vertebrate evolution. Mol Biol Evol 29:995–1004
Vijayaraj P, Kröger K, Reuter U, Windoffer R, Leube RE, Magin TM (2009) Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol 187:175–184
Wang J, Karabinos A, Schünemann J, Riemer D, Weber K (2000) The epidermal intermediate filament proteins of tunicates are distant keratins; a polymerisation-competent hetero coiled coil of the Styela D protein and Xenopus keratin 8. Eur J Cell Biol 79:478–487
Weber K, Plessmann U, Ulrich W (1989) Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J 8(11):3221–3227
Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M, Labouesse M (2011) A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471:99–103
Zhang QL, Zhang GL, Yuan ML, Dong ZX, Li HW, Guo J, Wang F, Deng XY, Chen JY, Lin LB (2018) A phylogeneticc framework and divergence history of cephalochordate Amphioxus. Front Physiol 9:1833. https://doi.org/10.3389/fphys.201801833
Zimek A, Stick R, Weber K (2003) Genes coding for intermediate filament proteins: common features and unexpected differences in the genomes of humans and the teleost fish Fugu rubripes. J Cell Sci 116:2295–2302
Acknowledgements
We would like to thank the team from Medirex, a.s. for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Georg Krohne
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Fig. S. Alignment of the protein sequences of the B. floridae (Bf), B. belcheri (Bb) and B. lanceolatum (Bl) IF proteins (panel a) D1, C1, C2 and N2, (panel b) E2 and E3, (panel c) E1 and Y1, (panel d) X1, (panel e) D1 and XP_019633116.1-krt-81, (panel f) N1, (panel g) N2, (panel h) N3 and (panel i) B1 and AѰ. Moreover, panels j a k present the phylogenetic tree generated by the maximum-likelihood and neighbour-joining analysis, respectively. Identical amino acids between the compared Branchiostoma proteins, are marked in bold, while dashes are used to optimize the sequence alignment. The arrowhead pointing down indicates the start and the end position of the corresponding IF rod coil 1A, 1B, col 2A and 2B segments, which are connected by linkers L1, L12 and L2. Asterisks below the rod segments mark the a and d positions of the heptad repeat pattern (panels a-i), while asterisks above the two C tail-sequences mark their two degenerate repeats, exhibiting a heptad repeat pattern participating in the formation of a double-stranded coiled coil dimer (panel a; for details see Karabinos 2013; Karabinos and Schünemann 2014). The “QxW” motifs of the ricin-type carbohydrate-binding domain of the C, D1 and N2 IF tail-sequences are boxed (panel a; for details see Fig. 1). A manual comparison of the BfD1 IF protein and the uncharacterized 1318-residue-long B. belcheri XP_019633116.1 sequence, which corresponds to the recently reported krt-81 gene (panel e), revealed the poorly conserved helix initiation motif in the proposed coil 1A segment and a pure sequence similarity of this sequence (revealing very poor heptads with many polar residues at the heptad positions a and d, 5 prolines and no conserved helix termination motif in the proposed coil 2 segment) to the rest of the compared prototypic Branchiostoma BfD1 cIF protein. Moreover, a unique long tail-like domain terminating with the nuclear envelope localisation domain KASH (Razafsky and Hodzic 2009), shared with the B. floridae (XP_035657632) and B. lanceolatum (CAH1252602.1) orthologs (data not shown), collectively indicate that this uncharacterized XP_019633116.1/krt-81 protein is not an IF. In panel h, both the BfN3 and BbN3 sequences have unusually long tail domains, so we think that identification of final amino acid sequences of these terminal sequences might await cloning of the corresponding full-length cDNAs. In panel i, positions of the BfB1 rod gene introns are marked by arrows pointing down, while positions of the four conserved rod BfAѰ-new introns, as well as the two novel rod BfAѰ-new introns, positioned to the divergent C-terminal part of this protein, are marked by arrows pointing up. The BfAѰ-new gene represents a revised version of the previously reported B. floridae sequence 81362-AѰ (named here as BfAѰ-old). As documented here, all four Branchiostoma sequences still retained a divergent C-terminus in which the last 13 residues VNLQINQHTNRGW of the original BfAѰ-old sequence have been replaced by 22 new residues, marked in the BfAѰ-new sequence (panel i; see text for details). In panel j is the phylogenetic tree generated by the maximum-likelihood analysis of the conserved rod domains of the IF proteins, derived from the B. floridae (Bf), B. belcheri (Bb) and B. lanceolatum (Bl) species (listed in Table 1) as well as the three protostomic sequences used as an outgroup. The bootstrap values (100 replications) are indicated above or below the internal nodes. The position of the Branchiostoma N4/N2, X1/N1, type I keratin, C1/C2, A/B, N3, type II keratin and the protostomic IF sequence groups are indicated. The arrows mark the monophyletic IF subgroups of the individual Branchiostoma species (see text for details). Note some phylogenetic evidence for a closer evolutionary relationship between the long protostomic-type N4 protein (marked by a double arrow) and the short chordate-type protein N2. Notably, this evolutionary N4/N2 relationship on the one hand, and the known ricin-type domain similarities of the N2, D1 and C IF tail sequences on the other hand, might potentially support an origin of all these five N4, N2, C1, C2 and D1 IF sequences from a common ancestor (see also Fig. 1, panel a above and the text for details). Note also a sister group relationship of the X1 and N1 with the type I keratins and the two keratin-like C IFs, in line with their keratin-like sequence features of the terminal domains (i.e. several glycine loops flanked by hydrophobic residues in panel d, g; for more details see Karabinos 2013). Finally, note that the phylogenetic attachment of the N3 sequences to the monophyletic A/B branch is also seen in the parallel neighbour-joining distance analysis (panel k below). However, none of the N1 to N4 positions had a bootstrap support, which indicates that any sister group relationship (e.g. N4/N2, N1/type I keratins, N3/A/B) drawn from this phylogeny, is interpreted as a provisional result. Finally, panel k presents the phylogenetic tree generated by the neighbour-joining analysis (for details, see panel j above and the text).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karabinos, A. The long protostomic-type cytoplasmic intermediate filament (cIF) protein in Branchiostoma supports the phylogenetic transition between the protostomic- and the chordate-type cIFs. Protoplasma 260, 1493–1500 (2023). https://doi.org/10.1007/s00709-023-01865-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-023-01865-3