Abstract
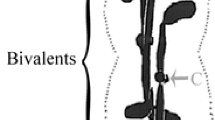
A “precocious” cleavage furrow develops and ingresses during early prometaphase in Mesostoma ehrenbergii spermatocytes (Forer and Pickett-Heaps Eur J Cell Biol 89:607-618, 2010). In response to chromosome movements which regularly occur during prometaphase and that alter the balance of chromosomes in the two half-spindles, the precocious furrow shifts its position along the cell, moving 2–3 μm towards the half cell with fewer chromosomes (Ferraro-Gideon et al. Cell Biol Int 37:892-898, 2013). This process continues until proper segregation is achieved and the cell enters anaphase with the cleavage furrow again in the middle of the cell. At anaphase, the furrow recommences ingression. Spindle microtubules (MTs) are implicated in various furrow positioning models, and our experiments studied the responses of the precocious furrows to the absence of spindle MTs. We depolymerized spindle MTs during prometaphase using various concentrations of nocodazole (NOC) and colcemid. The expected result is that the furrow should regress and chromosomes remain in the midzone of the cell (Cassimeris et al. J Cell Sci 96:9-15, 1990). Instead, the furrows commenced ingression and all three bivalent chromosomes moved to one pole while the univalent chromosomes, that usually reside at the two poles, either remained at their poles or moved to the opposite pole along with the bivalents, as described elsewhere (Fegaras and Forer 2018). The microtubules were completely depolymerized by the drugs, as indicated by immunofluorescence staining of treated cells (Fegaras and Forer 2018), and in the absence of microtubules, the furrows often ingressed (in 33/61 cells) at a rate similar to normal anaphase ingression (~ 1 μm/min), while often simultaneously moving toward one pole. Thus, these results indicate that in the absence of anaphase and of spindle microtubules, cleavage furrows resume ingression.








Similar content being viewed by others
References
Alsop GB, Zhang D (2003) Microtubules are the only structural constituent of the spindle apparatus required for induction of cell cleavage. J Cell Biol 162:383–390
Barr FA, Gruneberg U (2007) Cytokinesis: placing and making the final cut. Cell 131:847–860
Bonaccorsi S, Giansanti MG, Gatti M (1998) Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster. J Cell Biol 142:571–761
Capp A (1948) The life and times of the Shmoo. Pocket Books, New York
Cassimeris L, Rieder C, Rupp G, Salmon ED (1990) Stability of microtubule attachment to metaphase kinetochores in PtK1 cells. J Cell Sci 96:9–15
Choi HJ, Fukui M, Zhu BT (2011) Role of cyclin B1/Cdc2 up-regulation in the development of mitotic prometaphase arrest in human breast cancer cells treated with nocodazole. PLoS One 6(8):e24312. https://doi.org/10.1371/journal.pone.0024312
Cytrynbaum EN, Sommi P, Brust-Mascher I, Scholey JM, Mogilner A (2005) Early spindle assembly in Drosophila embryos: role of a force balance involving cytoskeletal dynamics and nuclear mechanics. Mol Biol Cell 16:4967–4981
Eggert US, Mitchison TJ, Field CM (2006) Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem 75:543–566
Fabian L, Xia X, Venkitaramani DV, Johansen KM, Johansen J, Andrew DJ, Forer A (2007) Titin in insect spermatocyte spindle fibers associates with microtubules, actin, myosin and the matrix proteins skeletor, megator and chromator. J Cell Sci 120:2190–2204
Fegaras E, Forer A (2018) Chromosomes selectively detach at one pole and quickly move towards the opposite pole when kinetochore microtubules are depolymerized in Mesostoma ehrenbergii spermatocytes. Protoplasma. https://doi.org/10.1007/s00709-018-1214-4
Ferraro-Gideon J, Hoang C, Forer A (2014) Meiosis-I in Mesostoma ehrenbergii spermatocytes includes distance segregation and inter-polar movements of univalent, and vigorous oscillations of bivalents. Protoplasma 251:127–143
Ferraro-Gideon J, Hoang C, Forer A (2013) Mesostoma ehrenbergii spermatocytes–a unique and advantageous cell for studying meiosis. Cell Biol Int 37(9):892–898
Forer A, Spurck T, Pickett-Heaps J, Wilson P (2003) Structure of kinetochore fibres in crane-fly spermatocytes after irradiation with an ultraviolet microbeam: neither microtubules nor actin filaments remain in the irradiated region. Cell Motil Cytoskeleton 56:173–192
Forer A, Pickett-Heaps J (2005) Fibrin clots keep non-adhering living cells in place on glass for perfusion or fixation. Cell Biol Int 29:721–730
Forer A, Pickett-Heaps J (2010) Precocious (pre-anaphase) cleavage furrows in Mesostoma spermatocytes. Eur J Cell Biol 89:607–618
Fuge H (1989) Rapid kinetochore movements in Mesostoma ehrenbergii spermatocytes: action of antagonistic chromosome fibres. Cell Motil Cytoskeleton 13:212–220
Fuge H (1987) Oscillatory movements of bipolar-oriented bivalent kinetochroes and spindle forces in male meiosis of Mesostoma ehrenbergii. Eur J Cell Biol 44:294–298
Glotzer M (2004) Cleavage furrow positioning. J Cell Biol 164(3):347–351
Gonczy P (2008) Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev 9:355–366
Grill SW, Goènczy P, Stelzer EHK (2001) Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409:630–633
Hiramoto Y (1975) Force exerted by the cleavage furrow of sea urchin eggs. Develop Growth Differ 17(1):27–38
Hoang C, Ferraro-Gideon J, Gauthier K, Forer A (2013) Methods for rearing Mesostoma ehrenbergii in the laboratory for cell biology experiments, including identification of factors that influence production of different egg types. Cell Biol Int 37(10):1089–1105
Husted L, Ruebush TK (1940) A comparative cytological and morphological study of Mesostoma ehrenbergii and Mesostoma ehrenbergii wardii. J Morphol 67:387–410
Ingber DE (1991) Integrins as mechanochemical transducers. Curr Biol 3:841–848
Ingber DE (1993) Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104:613–627
Ingber DE (2003a) Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116:1157–1173
Ingber DE (2003b) Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116:1397–1408
Johansen KM, Johansen J (2007) Cell and molecular biology of the spindle matrix. Int Rev Cytol 263:155–210
Johansen KM, Forer A, Yao C, Girton J, Johansen J (2011) Do nuclear envelope and intranuclear proteins reorganize during mitosis to form an elastic hydrogel-like spindle matrix? Chromosom Res 19:345–365
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4(4):253–265
Liu M, Post M (2000) Mechanochemical signal transduction in the fetal lung. J Appl Physiol 89:2078–2084
McIntosh K, Pickett-Heaps JD, Gunning BES (1995) Cytokinesis in Spirogyra: integration of cleavage and cell-plate formation. Int J Plant Sci 156:1–8
Mogessie B, Schuh M (2017) Actin protects mammalian eggs against chromosome segregation errors. Science 357:eaal 1647
Mollinedo F, Gajate C (2003) Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 8(5):413–450
Murthy K, Wadsworth P (2008) Dual role for microtubules in regulating cortical contractility during cytokinesis. J Cell Sci 121:2350–2359
Oakley HA (1984) Meiosis in Mesostoma ehrenbergii ehrenbergii (Turbellaria, Rhabdocoela). III. Univalent chromosome segregation during the first meiotic division in spermatocytes. Chromosoma 91(2):95–100
Pickett-Heaps JD (1991) Post-mitotic cellular reorganisation in the diatom Cymatopleura solea: the role of microtubules and the microtubule Centre. Cell Motil Cytoskel 18:279–292
Pickett-Heaps JD, Tippit DH, Andreozzi A (1978) Cell division in the pennate diatom Pinnularia. II Later stages in mitosis. Biol Cell 33:79–84
Pickett-Heaps JD, Tippit DH, Andreozzi A (1979) Cell division in the pennate diatom Pinnularia. V. Observations on living cells. Biol Cell 35:295–304
Pickett-Heaps JD, Tippit DH, Leslie R (1980) Light and electron microscopic observations on cell division in two large pennate diatoms, Hantzschia and Nitzschia. I. Mitosis in vivo. Eur J Cell Biol 21:1–11
Poxleitner MK, Dawson SC, Cande WZ (2008) Cell cycle synchrony in Giardia intestinalis cultures achieved by using nocodazole and aphidicolin. Eukaryot Cell 7(4):559–574
Quinlan RA, Schwarz N, Windoffer R, Richardson C, Hawkins T, Broussard JA, Green KJ, Leube RE (2017) A rim-and-spoke hypothesis to explain the biochemical roles for cytoplasmic intermediate filament networks. J Cell Sci 130:3437–3445
Rappaport R (1985) Repeated furrow formation from a single mitotic apparatus in cylindrical sand dollar eggs. J Exp Zool 234:167–171
Rappaport R (1981) Cytokinesis: cleavage furrow establishment in cylindrical sand dollar eggs. J Exp Zool 217:365–375
Rappaport R, Ebstein RP (1965) Duration of stimulus and latent periods preceding furrow formation in sand dollar eggs. J Exp Zool 158:373–382
Rappaport R (1961) Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool 148:81–89
Vasquez R, Howell B, Yvon AC, Wadsworth P, Cassimeris L (1997) Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell 8:973–985
von Dassow G, Verbrugghe KJC, Miller AL, Sider JR, Bement WM (2009) Action at a distance during cytokinesis. J Cell Biol 187(6):831–845
White CR, Frangos JA (2007) The shear stress of it all: the cell membrane and mechanochemical transduction. Phil Trans R Soc B 362:1459–1467
Wong R, Forer A (2003) Signalling between chromosomes in crane fly spermatocytes studied using ultraviolet microbeam irradiation. Chromosom Res 11:771–786
Yang H, Ganguly A, Cabral F (2010) Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. J Biol Chem 285(42):32242–32250
Yao C, Rath U, Maiato H, Sharp D, Girton J, Johansen KM, Johansen J (2012) A nuclear-derived proteinaceous matrix embeds the microtubule spindle apparatus during mitosis. Mol Biol Cell 23:3532–3541
Acknowledgements
This work was supported by research grants to AF from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Douglas Chandler
Electronic supplementary material
Supplementary video 1
cell treated with 20 μM NOC between 1259:54 and 13:00:01, when the images become out of focus. The bivalents oscillated up and back to the two spindle poles initially. After NOC was added, the oscillations ceased as the bivalents stretched out. The bivalent kinetochores facing the upper pole detached at around 13:01:16 and moved into the bottom half-spindle, leaving the univalents behind at the top pole. After these movements the cleavage furrow slowly ingressed and simultaneously moved towards the upper pole, forming a cell with two unequal parts, that looks like a ‘shmoo’. (MOV 6899 kb)
Supplementary video 2
cell treated with 20 μM NOC between frames 91–95, when the image was out of focus. [The time between each frame is 2 s.] As in video 1, after NOC the chromosomes stretched out; all bivalent kinetochores detached from the upper pole, starting at about frame 115, and moved to the bottom half-spindle together with the univalent from the upper pole. Subsequent to the chromosome movements the cleavage furrow ingressed and moved towards the upper pole, ending up at the cell periphery. (MOV 5514 kb)
Rights and permissions
About this article
Cite this article
Fegaras, E., Forer, A. Precocious cleavage furrows simultaneously move and ingress when kinetochore microtubules are depolymerized in Mesostoma ehrenbergii spermatocytes. Protoplasma 255, 1401–1411 (2018). https://doi.org/10.1007/s00709-018-1239-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-018-1239-8