Abstract
Cadmium (Cd) interferes with ascorbate and glutathione metabolism as it induces the production of reactive oxygen species (ROS), binds to glutathione due to its high affinity to thiol groups, and induces the production of phytochelatins (PCs) which use glutathione as a precursor. In this study, changes in the compartment specific distribution of ascorbate and glutathione were monitored over a time period of 14 days in Cd-treated (50 and 100 μM) Arabidopsis Col-0 plants, and two mutant lines deficient in glutathione (pad2-1) and ascorbate (vtc2-1). Both mutants showed higher sensitivity to Cd than Col-0 plants. Strongly reduced compartment specific glutathione, rather than decreased ascorbate contents, could be correlated with the development of symptoms in these mutants suggesting that higher sensitivity to Cd is related to low glutathione contents rather than low ascorbate contents. On the subcellular level it became obvious that long-term treatment of wildtype plants with Cd induced the depletion of glutathione and ascorbate contents in all cell compartments except chloroplasts indicating an important protective role for antioxidants in chloroplasts against Cd. Additionally, we could observe an immediate decrease of glutathione and ascorbate in all cell compartments 12 h after Cd treatment indicating that glutathione and ascorbate are either withdrawn from or not redistributed into other organelles after their production in chloroplasts, cytosol (production centers for glutathione) and mitochondria (production center for ascorbate). The obtained data is discussed in respect to recently proposed stress models involving antioxidants in the protection of plants against environmental stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contaminations of soils with heavy metals such as cadmium (Cd) are a severe problem for plant growth and development as they accumulate and negatively interfere with many physiological processes in plants. Environmental pollution by metals such as Cd became extensive in the late 19th and early 20th century as mining and industrial activities increased (Benavides et al. 2005; Gallego et al. 2012). Anthropogenic sources like zinc smelting, burning of fuel, waste incinerators, urban traffic, cement factories, and Cd as a by-product of phosphate fertilizers have led to Cd pollution in the environment (Sanità di Toppi and Gabbrielli 1999; DalCorso et al. 2008; Jozefczak et al. 2012). Although Cd is not an essential element, it is readily absorbed by the roots and transported through xylem and phloem to different parts of the plants (Mendoza-cózatl et al. 2008; DalCorso et al. 2008). Visible symptoms of Cd toxicity in plants are leaf roll, chlorosis, browning of root tips and reduced growth (Baryla et al. 2001; Schützendübel and Polle 2002; Chen et al. 2003; Gratão et al. 2005; Maughan et al. 2010). Inside the plant Cd leads to an alteration of the chloroplast ultrastructure, disturbs the synthesis of chlorophyll and carotenoids, inhibits the enzyme activity of the Calvin cycle and leads to CO2 deficiency due to stomatal closure, which cause the inhibition of photosynthesis (Ding et al. 2006; Ekmekci et al. 2008; He et al. 2008; Gratão et al. 2009; Bouzon et al. 2012). Additionally, Cd causes lipid peroxidation, disrupts cell transport processes, reduces the uptake of essential mineral nutrients, reduces the activity of various enzymes and is responsible for chromosomal aberrations (Clemens et al. 2001; Schützendübel and Polle 2002; Ding et al. 2006; Sanità di Toppi et al. 2008; Gratão et al. 2012). Indirectly, high concentrations of Cd lead to oxidative stress which activates the accumulation of anti-oxidative defense enzymes and antioxidants like ascorbate and glutathione (Sanità di Toppi and Gabbrielli 1999; Hegedüs et al. 2001; Gratão et al. 2005; Paradiso et al. 2008; Ekmekci et al. 2008; Xu et al. 2008; DalCorso et al. 2008; Gill and Tuteja 2010; Yadav 2010; Monteiro et al. 2011; Gallego et al. 2012; Shan et al. 2012; Zelinova et al. 2013) which detoxify reactive oxygen species (ROS) either through reactions catalyzed by peroxidases or through the ascorbate glutathione cycle (Foyer and Noctor 2005; Iannone et al. 2010; Foyer and Noctor 2011; Noctor et al. 2012). In addition, Cd has a high affinity to thiol groups and forms complexes with reduced glutathione which are then transported into vacuoles. Glutathione is also able to protect proteins by reversible binding to their SH groups which protects them from oxidation by ROS and inhibits the binding of Cd (Rauser 2001; Maksymiec and Krupa 2006; Semane et al. 2007; DalCorso et al. 2010; Jozefczak et al. 2012). On top glutathione serves as a direct precursor of phytochelatins (PCs), which together with metallothioneins detoxify Cd by complexation, transportation, and deposition in the vacuole (Akhter et al. 2012).
Considering the importance of ascorbate and glutathione in the protection against Cd changes in the contents of these antioxidants are well documented in Cd-treated plants. After treatment with 10 μM Cd sulfate for 24 h, Arabidopsis thaliana plants showed reduced ascorbate contents in roots (Smeets et al. 2009) and leaves (Keunen et al. 2013). While experiments with Brassica juncea showed increased levels of ascorbate in roots after Cd treatment (Mohamed et al. 2012), decreased ascorbate levels were found after long-term Cd exposure of Ceratophyllum demersum (Aravind and Prasad 2005), Brassica campestiris (Anjum et al. 2008) and Bechmeria nivea (L.) Gaud (Liu et al. 2007). Glutathione levels in Cd-treated plants were found to be decreased, e.g., in soybean leaves (Noriega et al. 2012), in cotyledons and embryonic axes of pea seed (Smiri et al. 2011), in Cd sensitive rice seedlings (Chao et al. 2011), in B. campestiris leaves (Anjum et al. 2008), and within glandular trichomes of Cucurbita pepo (Kolb et al. 2010). Other studies revealed either unchanged glutathione contents for example within Cd tolerant rice seedlings (Chao et al. 2011), or increased glutathione levels like in B. juncea shoots and roots (Mohamed et al. 2012), in leaves of Bechmeria nivea (Liu et al. 2007) and in Arabidopsis plants (Wójcik and Tukiendorf 2011). Even though these investigations paint a clear picture about the importance and functions of ascorbate and glutathione in the protection against Cd the results of these studies differ in time points, Cd concentration, plant species as well as plant organs and tissues. In particular, the situation during Cd exposure over a period of 14 days in Arabidopsis remains unclear. As stress responses in plants strongly depend on plant species, the state of acclimation and adaptation, severity and duration of stress (Tausz et al. 2004; Gratão et al. 2008; Kranner et al. 2010), it is important to monitor different severities of stresses at different time points in one plant species in order to get a deeper insight into the stress response of a plant. Additionally, Cd-induced stress responses in plants have mainly been investigated by using biochemical methods in order to investigate ascorbate and glutathione contents in whole leaves or organs (Liu et al. 2007; Anjum et al. 2008; Chao et al. 2011; Smiri et al. 2011; Wójcik and Tukiendorf 2011; Noriega et al. 2012; Mohamed et al. 2012). Thus, results about the situation concerning glutathione or ascorbate contents are rarely available for individual cell compartments (Kolb et al. 2010). Since Cd enters the cytosol first and affects this cell compartment stronger than others compartment specific data of ascorbate and glutathione contents could give valuable information about the subcellular importance of ascorbate and glutathione in the protection against Cd.
Thus, the aim of this study was to investigate the compartment-specific distribution of ascorbate and glutathione in Arabidopsis plants during Cd exposure. Different concentrations and time points of Cd treatment were investigated over a period of 14 days in order to clarify the dynamic compartment specific protection of these key antioxidants against Cd exposure. Additionally, we compared the situation in the ascorbate and glutathione deficient mutant vtc2-1 (60 % less ascorbate than Col-0; Zechmann et al. 2011c) and pad2-1 (80 % less glutathione than Col-0; (Parisy et al. 2006; Zechmann et al. 2008), respectively, in order to clarify how low ascorbate and glutathione contents contribute towards Cd sensitivity.
Materials and methods
Plant material and growth conditions
After stratification for 4 days at 4 °C, seeds of A. thaliana accession Col-0 and two Arabidopsis mutants (pad2-1, vtc2-1) were grown in a mixture of vermiculite and sand (2:1) in growth chambers with a 10/14 h day/night photoperiod. Temperatures were 22 °C during daytime and 18 °C at night. The relative humidity was 60 % and the relative soil water content was kept at 100 %. The light intensity was 150 μM m−2 s−1.
For Cd treatment, 6- to 8-week-old plants were removed from the pots and were gently washed with water to eliminate vermiculite and sand from the roots. The plants were transferred into plastic dishes (1,500 ml) covered with a nylon mesh that held up the stems and leaves whereas the roots immersed into the solution. Oxygen was supplied to the solution through an aquarium pump. For treatment the dishes were filled either with nutrient solution as control or nutrient solution mixed with Cd sulfate (50 and 100 μM). Cd treatment was performed for different time points (12 h, 24 h, 48 h, 96 h, 7 days and 14 days) with plants of different age so that by the time of harvesting, all plants were 8 weeks old. The solution contained 5 mM KNO3, 1 mM KH2PO4, 2 mM Mg(NO3)2⋅6H2O, 2.5 mM CaSO4⋅2H2O, 1 mM MgSO4⋅7H2O, 70 μM EDTA-FeNa, 4 mM Ca(NO3)2⋅4H2O, 0.9 μM ZnCl2, 30 μM H3BO3, 0.9 μM CuCl2⋅2H2O, 0.5 μM MoO3, 20 μM MnCl2⋅4H2O and was as well as the Cd solution changed every 4 days. The pH value of the solution was adjusted to 6.5.
Sample preparation for transmission electron microscopy
Two hours after the onset of the light period the center of three different leaves (always taken from the fourth rosette) from each sample were cut into 1.5-mm2 pieces on a modeling wax plate in a drop of 2.5 % paraformaldehyde/0.5 % glutardialdehyde in 0.06 M Sørensen phosphate buffer (pH 7.2). The samples were then transferred into glass vials filled with the same fixative for 90 min at room temperature (RT) and were then dehydrated with increasing concentrations of acetone (50 %, 70 %, 90 %). Subsequently, specimens were infiltrated with increasing concentrations of LR-White resin (30 %, 60 %, 100 %; London Resin Company Ltd., Berkshire, UK) mixed with acetone (90 %) for at least 3 h at each step at RT. Finally, the samples where embedded in pure, fresh LR-White resin and polymerized at 50 °C for 48 h in small plastic cups under anaerobic conditions. Ultrathin sections of about 80 nm were generated by cutting the samples with a Reichert Ultracut S ultramicrotome (Leica Microsystems, Wetzlar, Germany).
Immunogold labeling of ascorbate and glutathione
Cytohistochemical investigation were performed as described previously (Zechmann et al. 2011a, b, c). Ultrathin sections were blocked with 2 % bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) in phosphate buffered saline (pH 7.2). Subsequently the samples were treated with the primary antibodies against ascorbate (anti-ascorbate rat polyclonal IgG; Abcam plc, Cambridge, UK) diluted 1:300 in phosphate buffered saline containing 1 % BSA and glutathione (anti-glutathione rabbit polyclonal IgG, Millipore Corp., Billerica, Ma, USA) diluted 1:50 in phosphate buffered saline with 1 % goat serum for 2 h at RT. Then the specimens where rinsed three times with phosphate buffered saline containing 1 % BSA and treated with a 10-nm gold-conjugated secondary antibody (goat anti-rat IgG for ascorbate labeling and goat anti-rabbit IgG [British BioCell International, Cardiff, UK] for glutathione labeling) diluted 1:100 for ascorbate and 1:50 for glutathione in phosphate-buffered saline containing 1 % BSA for 90 min at RT. Next, the samples were washed two times in distilled water and post-stained with uranyl acetate (2 % dissolved in aqua bidest) for 20 s. The samples were analyzed with a Philips CM10 transmission electron microscope where micrographs of randomly photographed immunogold-labeled sections at 8,900-fold magnification were taken. Micrographs of randomly photographed immunogold labeled sections were digitized and gold particles were counted automatically using the software package Cell D with the particle analysis tool (Olympus, Life and Material Science Europa GmbH, Hamburg, Germany) in different visually identified and manually traced cell structures (mitochondria, plastids, nuclei, peroxisomes, the cytosol, vacuoles). Unspecific background labeling was determined on the sections (outside the specimen) and subtracted from the values obtained in the sample. A minimum of 20 (peroxisomes and vacuoles) to 60 (other cell structures) sectioned cell structures of at least 15 different cells were analyzed for gold particle density per sample. For statistical evaluation at least two different samples for each treatment (n = 20 for peroxisomes and vacuoles, n = 60 for other cell compartments) were analyzed to gain the number of gold particles per μm2 per compartment. Differences in gold particle density of Cd-treated and control samples were calculated in percent. Control plants showed similar (not significantly different) subcellular ascorbate and glutathione contents throughout the experiment. Thus, a mean of the determined subcellular ascorbate and glutathione contents was calculated, defined as control and used for calculating significant differences between Cd-treated and control samples. The obtained data were statistically evaluated using Statistica (Stat-Soft Europe, Hamburg, Germany). For statistical analyses, the Mann–Whitney U was applied.
Several negative controls were made to support the specificity of the immunogold procedure. Negative controls were treated either with (1) gold conjugated secondary antibody (goat anti-rat IgG for ascorbate and goat anti-rabbit IgG for glutathione) without prior incubation of the section with the primary antibodies, (2) non-specific secondary antibody (goat anti rabbit IgG for ascorbate and goat anti rat IgG for glutathione), (3) preimmune serum instead of the primary antibody and (4) primary antibody against ascorbate and glutathione pre-adsorbed with an excess of reduced and oxidized ascorbate and glutathione, respectively, for 2 h prior to labeling of the sections. For the latter, a solution containing either 10 mM of ascorbic acid, dehydroascorbic acid, reduced or oxidized glutathione was incubated with or without 0.5 % glutardialdehyde for 1 h. When glutardialdehyde was used then its excess was saturated by incubation for 30 min in a solution of 1 % (w/v) BSA. The resulting solutions were both used in independent experiments to saturate the anti-ascorbate and glutathione antibodies for 2 h prior to its use in the immunogold labeling procedure described above. Labeling on sections treated as negative controls showed no or only very little gold particles bound to ascorbate and glutathione which was similar to previous results obtained by using the same methods in different plant species (Zechmann and Müller 2010; Zechmann et al. 2011c). We have shown in previous reports that the antibody against glutathione binds to free reduced and oxidized glutathione and that it does not bind to glutathione conjugated with monochlorobimane through the SH group and glutationylated proteins (Zechmann et al. 2008; Queval et al. 2011). Additionally, it does not bind to PCs as it binds to the amino group and both carbonyl groups of glutathione — the latter are not available for the antibody in PCs (personal communication with Signature Immunologics Inc.). The glutathione and ascorbate status in the glutathione and ascorbate deficient mutants used in this study (pad2-1 and vtc2-1, respectively) has been studied extensively in previous studies and showed a good correlation between data obtained on the subcellular level and data obtained on the whole leaf level with biochemical methods (Parisy et al. 2006; Zechmann et al. 2008; Zechmann and Müller 2008; Zechmann et al. 2011c). The immunogold localization of ascorbate in mutants deficient in ascorbate (vtc2-1 and vtc2-2) revealed a strong decrease of subcellular ascorbate specific labeling between 50 % and 60 % when compared to A. thaliana Col-0 plants. This data correlated well with biochemical measurements which revealed a similar decrease of ascorbate contents in whole leaves of these mutants (Zechmann et al. 2011c). The specificity and accuracy of the immunogold labeling method for glutathione was demonstrated on glutathione deficient mutants pad2-1 and rml1, which both showed a strong decrease of compartment-specific glutathione labeling of up to 91 % and 98 %, respectively. This data correlated well with biochemical measurements of glutathione in these mutants revealing a similar decrease in whole leaves of these mutants (Vernoux et al. 2000; Cairns et al. 2006; Parisy et al. 2006).
Results
Visual symptoms
Leaves of plants that were treated without Cd did not develop chlorosis and necrosis (Figs. 1a,e,i and 2a,e,i). First negative effects of Cd treatment in the form of local chlorotic spots could be found on wildtype plants 14 days after the treatment with 50 μM Cd (Fig. 1d). Leaves of pad2-1 mutants showed first signs of chlorosis and necrosis after 7 days of 50 μM Cd treatment which became more severe 14 days after treatment with 50 μM Cd (Fig. 1g, h.). vtc2-1 mutants showed minor signs of chlorosis after 7 days and strong chlorotic spots 14 days after the treatment with 50 μM Cd (Fig. 1k, l). After treatment with 100 μM Cd wildtype plants developed chlorosis after 7 days, which became more severe 14 days after Cd treatment (Fig. 2c,d). Leaves of pad2-1 mutants showed first signs of chlorosis after 96 h (Fig. 2f) and both chlorosis and necrosis after 7 days of 100 μM Cd treatment (Fig. 2g). Large necrotic areas and chlorotic spots were visible on leaves of pad2-1 mutants 14 days after Cd treatment (Fig. 2h). Leaves of vtc2-1 mutants showed first signs of chlorosis after 96 h (Fig. 2j) of treatment with 100 μM Cd (Fig. 2j). Chlorosis and necrosis became more severe 7 and 14 days after 100 μM of Cd treatment but did not reach the extent observed in pad2-1 and Col-0 (Fig. 2k,l).
Representative images of leaves from Arabidopsis thaliana Col-0 (a–d), and the mutants pad2-1 (e–h) and vtc2-1 (i–l) treated with 50 μM of Cd for 0 (a, e, i), 96 h (b, f, j), 7 days (c, g, k) and 14 days (d, h, l). First signs of Cd-induced alterations such as chlorotic lesions could be observed in the mutants 7 days (g, k) after Cd treatment which became more prominent and covered larger areas 14 days (h, l) after Cd treatment. Wildtype plants showed first signs of chlorotic lesions starting at 14 days after Cd treatment (d)
Representative images of leaves from Arabidopsis thaliana Col-0 (a–d), and the mutants pad2-1 (e–h) and vtc2-1 (i–l). Leaves derived from plants which were treated without Cd for 14 days (a, e, i) or with 100 μM of Cd for 96 h (b, f, j), 7 days (c, g, k) and 14 days (d, h, l). First signs of Cd-induced alterations such as the appearance of chlorosis and necrosis could be observed in the mutants 96 h (f, j) after Cd treatment which became more prominent and covered larger areas 7 days (g, k) and 14 days (h, l) after Cd treatment. Wildtype plants showed first signs of chlorotic lesions starting at 7 days after Cd treatment (c) which covered larger areas 14 days after Cd treatment (d)
Immunogold labeling of ascorbate
50 μM Cd
The subcellular distribution of ascorbate in control plants was similar in most cell compartments to what has been reported previously (Zechmann et al. 2011c). Wildtype plants and pad2-1 mutants contained similar levels of ascorbate in all cell compartments whereas the vtc2-1 mutant contained between −50 % and −75 % less ascorbate (vacuoles and nuclei, respectively) than the wildtype (Table 1). These results are similar to what has been described in previous studies which revealed that the vtc2-1 mutant contained between −50 and −60 % less ascorbate than wildtype plants (Zechmann et al. 2011c).
Mitochondria of wildtype plants showed significant decreased levels of gold particles bound to ascorbate after 12 h (−49 %), 24 h (−52 %) and 48 h (47 %) and significant increased levels after 7 days (80 %) after treatment with 50 μM Cd (Fig. 3). In chloroplasts decreased amounts of gold particles bound to ascorbate could be observed after 48 h (−18 %), whereas increased ascorbate levels could be observed after 96 h (187 %) of Cd treatment when compared to the control. The amount of gold particles in nuclei significantly decreased after exposure to 50 μM Cd for 12 h (−31 %) and 14 days (−36 %) and showed increased levels after 96 h (82 %) compared to the control. In the cytosol decreased ascorbate levels could be found after 24 h (−43 %) and 48 h (−48 %) after treatment with 50 μM Cd. In peroxisomes significantly decreased ascorbate levels could be observed after 12 h (−51 %) and increased levels after 96 h (147 %). In the vacuole, the numbers of gold particles bound to ascorbate showed a decrease after 7 days (−46 %) whereas no change was found at the other time points when compared to the control (Fig. 3, Fig. S1).
Compartment specific changes in ascorbate labeling density at different time points after the treatment of 50 μM Cd. Graphs show the percentage of increase and decrease of gold particles bound to ascorbate per μm2 in mesophyll cells of Arabidopsis thaliana Col-0 plants (black squares) and the Arabidopsis mutants pad2-1 (red circles) and vtc2-2 (blue triangles). Differences were calculated by comparing Cd-treated samples with control samples which were treated with nutrient solution without Cd (values can be found in Table 1). n>20 for peroxisomes and vacuoles and n>60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann–Whitney U-test; *, ** and *** indicate significance at the 0.05, 0.01 and 0.001 levels of confidence, respectively
Mitochondria of pad2-1 mutants showed significantly decreased ascorbate levels after 12 h (−38 %) and 14 days (−49 %) and increased levels after 48 h (44 %) after treatment with 50 μM Cd (Fig. 3). The amount of gold particles bound to ascorbate in chloroplasts significantly decreased after 12 h (−62 %), whereas after 48 h (58 %) and 7 days (60 %) an increase of gold particles compared to the control could be observed. In nuclei, increased amounts of gold particles bound to ascorbate could be observed after 48 h (51 %) after Cd treatment. In the cytosol, the ascorbate levels decreased significantly (between −43 % and −52 %) after 12, 24 and 96 h, whereas after 48 h significantly increased ascorbate levels (26 %) could be observed. The amount of gold particles bound to ascorbate in peroxisomes significantly increased after exposure to Cd for 12 h (12 %), 48 h (127 %) and 96 h (10 %) and decreased after 24 h (43 %) and 14 days (68 %). The vacuole showed significant increased ascorbate levels after Cd treatment for 24 h (42 %), 48 h (66 %), 96 h (32 %) and 7 days (30 %) compared to the control (Fig. 3, Fig. S2).
In the mitochondria of vtc2-1 mutants, the labeling density was significantly increased after 48 h (112 %), whereas decreased levels of ascorbate were found after 12 h (−36 %) and 14 days (−70 %) after treatment with 50 μM Cd (Fig. 3). In chloroplasts, the number of gold particles bound to ascorbate was significantly decreased after 12 h (−39 %) and 7 days (44 %) whereas after 48 h an increase of 100 % could be observed. The amount of gold particles bound to ascorbate in nuclei was significantly increased after 48 h (89 %) and 96 h (62 %) whereas decreased levels could be observed after 14 days (−70 %) after treatment with 50 μM Cd. In the cytosol ascorbate levels significantly decreased after 12 h (−36 %) and 14 days (−47 %) and increased after 48 h (89 %) compared to the control. Generally the ascorbate levels in peroxisomes were similar to control levels except after 48 h after Cd treatment where an increase of 82 % could be observed. The vacuole showed increased ascorbate levels after 48 h (39 %) and 96 h (33 %) compared to the control (Fig. 3, Fig. S3).
100 μM Cd
In the mitochondria of wildtype plants, the labeling density of ascorbate was significantly decreased after 24 h (−42 %) and 48 h (−51 %) after treatment with 100 μM Cd (Fig. 4). In plastids decreased amounts of gold particles bound to ascorbate could be observed after 12 h (−31 %), 24 h (−37 %) and 7 days (−24 %), whereas significant increased levels was found after 96 h (48 %) and 14 days (30 %) when compared to the control. In nuclei, the gold particle density bound to ascorbate decreased significantly after 12 h (−37 %) and 24 h (−32 %). Generally, the ascorbate levels in the cytosol where significantly decreased (up to −43 %). In peroxisomes, the numbers of gold particles bound to ascorbate increased after 96 h (109 %) compared to the control whereas after 12 h (−50 %), 24 h (−47 %) and 14 days (−64 %) significant decreased ascorbate levels could be observed. The vacuole showed decreased levels of ascorbate after 12 h (−33 %), 24 h (47 %) and 7 days (−36 %) after treatment with 100 μM Cd (Fig. 4, Fig. S1).
Compartment specific changes in ascorbate labeling density at different time points after the treatment of 100 μM Cd. Graphs show the percentage of increase and decrease of gold particles bound to ascorbate per μm2 in mesophyll cells of Arabidopsis thaliana Col-0 plants (black squares) and the Arabidopsis mutants pad2-1 (red circles) and vtc2-2 (blue triangles). Differences were calculated by comparing Cd-treated samples with control samples which were treated with nutrient solution without Cd (values can be found in Table 1). n>20 for peroxisomes and vacuoles and n>60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann–Whitney U-test; *, ** and *** indicate significance at the 0.05, 0.01 and 0.001 levels of confidence, respectively
Mitochondria of pad2-1 mutants showed after 12 h (−63 %), 48 h (−43 %), 7 days (−36 %) and 14 days (−37 %) significant decreased levels of ascorbate after treatment with 100 μM Cd (Fig. 4). In plastids, the ascorbate levels were generally decreased (between −13 % and −66 %) when compared to the control. In nuclei, significant decreased amounts of gold particles bound to ascorbate could be observed after 48 h (−27 %). In the cytosol, the numbers of gold particles where decreased significantly after 12 h, 48 h, 96 h, 7 days and 14 days (between −36 % and −65 %). In peroxisomes, the labeling density of ascorbate showed no significant differences to the control. In the vacuole, significant decreased ascorbate levels could be observed after 12 h (−23 %) and 7 days (24 %), whereas after 24 h the number of gold particles bound to ascorbate increased up to 29 % (Fig. 4, Fig. S2).
After exposure to 100 μM Cd, mitochondria of vtc2-1 mutants showed a significant increase of ascorbate levels after 48 h (45 %) whereas after 7 days (−51 %) and 14 days (−54 %) significant decreased levels compared to the control could be observed (Fig. 4). In plastids the amounts of gold particles bound to ascorbate significantly decreased after 12 h (−34 %) and 24 h (−44 %), whereas after 48 h increased ascorbate levels compared to the control could be observed (99 %). In nuclei the labeling density of ascorbate was significantly increased after 96 h (73 %), whereas decreased levels were found after 14 days (−56 %). In the cytosol the numbers of gold particles bound to ascorbate significantly increased after 48 h (49 %) and 96 h (24 %), whereas after 7 days (−46 %) and 14 days (−37 %) decreased ascorbate levels could be observed. In peroxisomes the labeling density of ascorbate increased significantly after 24 h (109 %) and 48 h (126 %) compared to the control. In the vacuole a significant higher labeling density of ascorbate could be observed after 48 h (58 %) compared to the control (Fig. 4, Fig.S3).
Immunogold labeling of glutathione
50 μM Cd
The subcellular distribution of glutathione in control plants was similar in most cell compartments to what has been reported previously (Zechmann and Müller 2010). Wildtype plants and vtc2-1 mutants contained similar levels of glutathione in all cell compartments whereas the pad2-1 mutant contained between −87 % and −71 % less glutathione (nuclei and chloroplasts, respectively) than the wildtype (Table 2). In mitochondria, the pad2-1 mutant contained similar levels of glutathione when compared to the wildtype and the vtc2-1 mutant (Table 2). These results are similar to what has been described in previous studies which revealed that mitochondria of the pad2-1 mutant contained wildtype glutathione levels whereas all other cell compartments contained up to −90 % less glutathione than the wildtype (Zechmann et al. 2008). Additionally, it has been shown in previous studies that vtc2-1 mutants contain similar glutathione levels when compared to the wildtype (Fernandez-Garcia et al. 2009).
Mitochondria of wildtype plants showed a significant increase of gold particles bound to glutathione after 12 h (17 %), 24 h (22 %), 48 h (69 %), 7 days (30 %) and 14 days (43 %) treatment with 50 μM Cd (Fig. 5). In chloroplasts, elevated glutathione levels between 32 % and 124 % compared to the control could be observed at all time points. In nuclei, increased amounts of gold particles bound to glutathione could be observed after 12 h (42 %), 24 h (63 %), 48 h (89 %), 96 h (84 %) and 14 days (90 %) compared to the control. In the cytosol increased glutathione levels were found over the whole experiment (between 45 % and 134 %) after exposure to 50 μM Cd. The amount of gold particles bound to glutathione in peroxisomes significantly increased after exposure to Cd for 12 h (104 %), 24 h (53 %), 48 h (153 %) and 7 days (23 %; Fig. 5, Fig. S4).
Compartment specific changes in glutathione labeling density at different time points after the treatment of 50 μM Cd. Graphs show the percentage of increase and decrease of gold particles bound to glutathione per μm2 in mesophyll cells of Arabidopsis thaliana Col-0 plants (black squares) and the Arabidopsis mutants pad2-1 (red circles) and vtc2-2 (blue triangles). Differences were calculated by comparing Cd-treated samples with control samples which were treated with nutrient solution without Cd (values can be found in Table 2). n>20 for peroxisomes and vacuoles and n>60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann–Whitney U-test; *, ** and *** indicate significance at the 0.05, 0.01 and 0.001 levels of confidence, respectively
After exposure to 50 μM Cd, mitochondria (between −24 % and −90 %), chloroplasts (between −48 % and −78 %) and the cytosol (between −34 % and −81 %) of pad2-1 mutants showed significantly decreased glutathione levels at all time points when compared to the control (Fig. 5). Generally, glutathione labeling in nuclei was significantly decreased (between −23 % and −86 %) until 14 days, where surprisingly a significant increase of 62 % could be observed (Fig. 5, Fig. S5). Peroxisomes showed significantly decreased amounts of gold particles bound to glutathione after Cd treatment for 12 h (−65 %), 24 h (−80 %), 48 h (−81 %), 7 days (−74 %) and 14 days (−52 %).
In mitochondria of vtc2-1 mutants the labeling density was significantly increased after 24 h (30 %), whereas decreased levels were found after 96 h (−46 %) of Cd treatment (Fig. 5). No change was found at the other time points. In chloroplasts the labeling density of glutathione significantly decreased (between −54 % and −95 %) over the whole experiment when compared to the control. Nuclei of vtc2-1 mutants showed significantly higher amounts of gold particles bound to glutathione after Cd treatment for 24 h (75 %), 7 days (43 %) and 14 days (26 %) whereas decreased levels were found after 96 h (−33 %). In cytosol, increased amounts of gold particles bound to glutathione could be observed after 24 h (107 %) and 7 days (31 %) compared to the control whereas a significant decrease of −68 % could be analyzed after Cd treatment for 96 h. In peroxisomes, the gold particle density decreased −29 % and −66 % after Cd treatment for 12 h and 96 h whereas after 24 h the number of gold particles bound to glutathione increased up to 58 % compared to the control (Fig. 5, Fig. S6).
100 μM Cd
Mitochondria of wildtype plants showed significantly higher amounts of gold particles bound to glutathione after treatment with 100 μM Cd for 48 h (54 %) and 7 days (50 %) whereas decreased levels were found after 12 h (−83 %) and 14 days (−30 %, Fig. 6). In chloroplasts, significantly decreased glutathione levels could be observed after 12 h (−84 %) and increased levels after 48 h (68 %), 96 h (58 %), 7 days (137 %) and 14 days (90 %) when compared to the control. After 12 h the gold particle density in nuclei was significantly decreased (−69 %) whereas after 48 h, 96 h and 7 days an increase between 62 % and 94 % compared to the control could be observed. The cytosol showed significantly decreased amounts of gold particles bound to glutathione after Cd treatment for 12 h (−73 %) and 14 days (−25 %) and increased amounts after 48 h (70 %), 96 h (78 %) and 7 days (165 %) compared to the control. Twelve hours and 14 days after exposure to Cd, the labeling density in peroxisomes significantly decreased (between 45 % and 68 %), while after 24 h, 48 h and 7 days increased amounts of gold particles (between 50 % and 149 %) could be observed (Fig. 6, Fig. S4).
Compartment specific changes in glutathione labeling density at different time points after the treatment of 100 μM Cd. Graphs show the percentage of increase and decrease of gold particles bound to glutathione per μm2 in mesophyll cells of Arabidopsis thaliana Col-0 plants (black squares) and the Arabidopsis mutants pad2-1 (red circles) and vtc2-2 (blue triangles). Differences were calculated by comparing Cd-treated samples with control samples which were treated with nutrient solution without Cd (values can be found in Table 2). n>20 for peroxisomes and vacuoles and n>60 for other cell structures. Data are means with standard errors. Significant differences were calculated using the Mann–Whitney U-test; *, ** and *** indicate significance at the 0.05, 0.01 and 0.001 levels of confidence, respectively
Significantly decreased glutathione labeling could be observed in mitochondria (between −25 % and −90 %), chloroplasts (between −59 % and −83 %) and cytosol (between −41 % and 80 %) of pad2-1 mutants at all time points after exposure to 100 μM Cd. In nuclei and peroxisomes, the numbers of gold particles bound to glutathione were generally decreased (between −31 % and −85 % in nuclei and between −63 % and −88 % in peroxisomes), and no change was found after 96 h compared to the control (Fig. 6, Fig. S5).
At all time points the exposure to Cd significantly decreased labeling density in mitochondria (between −16 % and −49 %) and the cytosol (between −22 % and −47 %) in vtc2-1 mutants. Generally, glutathione labeling in chloroplasts was significantly decreased for the first 7 days (between −39 % and −69 %) whereas 14 days after Cd exposure an increase of 49 % compared to the control could be observed. The amount of gold particles bound to glutathione in nuclei significantly decreased after exposure to Cd for 48 h (−32 %), 96 h (−28 %) and 7 days (−40 %). In peroxisomes significantly decreased glutathione levels (between 40 % and 56 %) were found after the exposure to Cd for 24 h, 96 h, 7 days and 14 days (Fig. 6; Fig. S6).
Discussion
In general, pad2-1 and vtc2-1 mutants were more sensitive to the exposure to Cd than wildtype plants. After the exposure to Cd both mutants showed symptoms earlier than the wildtype and developed stronger signs of chlorosis and necrosis which covered larger areas of the leaves. pad2-1 mutants which contain up to 90 % less glutathione than wildtype plants (Parisy et al. 2006; Zechmann et al. 2008) showed even higher sensitivity to Cd exposure than vtc2-1 mutants as symptoms occurred earlier and stronger than in vtc2-1 mutants. Throughout the experiment, the higher sensitivity of pad2-1 and vtc2-1 mutants could be correlated to much lower glutathione contents when compared to Col-0, which indicates the importance of glutathione for the protection of plants against Cd stress. Similar results as those observed in this study have also been obtained for the glutathione deficient cad2-1 mutant and plants with artificially reduced glutathione contents which also showed higher Cd sensitivity due to low glutathione contents (Howden et al. 1995; Wójcik and Tukiendorf 2011). It was also shown that increased glutathione contents in the cdr3-1D mutants are partially required for enhanced Cd resistance (Wang et al. 2011). Even though higher Cd sensitivity of ascorbate deficient mutants has not been documented yet in the literature, such effects seem likely as several physiological studies demonstrated a correlation between low tissue concentrations of ascorbate and glutathione and Cd sensitivity (Wójcik and Tukiendorf 2011). Thus, we can conclude that higher Cd sensitivity of glutathione and ascorbate deficient mutants are due to lower antioxidative capacity of these plants when compared to wildtype plants.
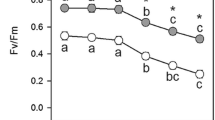
In this respect, it is interesting that glutathione contents in all cell compartments of wildtype plants followed recently proposed antioxidative stress models (Tausz et al. 2004; Kranner et al. 2010) which correlated well with symptom development on the leaves (Fig. 7). In wildtype plants exposed to 50 μM Cd which showed only minor symptoms 14 days after treatment, glutathione contents increased immediately after Cd exposure and remained at high levels until the end of the experiment indicating an acclimation effect of these plants to the exposure to Cd similar to what was described by Tausz et al. (2004). Ascorbate contents reacted in these plants with an initial decrease in ascorbate contents (initial alarming phase), a strong increase in ascorbate contents (resistance phase) and a strong decrease in ascorbate contents (final exhaustion phase; Fig. 7). As strong symptoms remain minor or absent in these plants, these results indicate that high glutathione contents rather than high ascorbate protect plants from Cd induced symptoms in the long term. Wildtype plants exposed to 100 μM showed a typical bell shaped stress response curve (Kranner et al. 2010) with an immediate decrease in glutathione and ascorbate contents which indicates an excessive demand for antioxidants immediately after the exposure to Cd (Fig. 7). Such a reaction of plants to excess Cd seems likely as Cd has a high affinity to thiol groups and would bind to reduced glutathione present in the cytosol after entering the cells (Semane et al. 2007; DalCorso et al. 2008; Jozefczak et al. 2012). Additionally, glutathione is used for the increased production of PCs which are also involved in the detoxification of Cd (DalCorso et al. 2008; Jozefczak et al. 2012). On top both antioxidants play important roles to prevent the accumulation of ROS which is commonly observed during the exposure of plants to heavy metals (Gill and Tuteja 2010; Yadav 2010). Nevertheless, considering that Cd enters the cytosol first and is not relocated into other cell compartments except vacuoles (Van Belleghem et al. 2007) it is interesting that glutathione and ascorbate decreased in all other cell compartments at similar rates. These results are similar to what we have observed for glutathione contents in previous studies (Kolb et al. 2010) and indicates that glutathione and ascorbate are either withdrawn from the other cell compartments to assuage the higher demand of glutathione in the cytosol or that less freshly produced ascorbate and glutathione get transported from the origin of synthesis (e.g., mitochondria for ascorbate [Bartoli et al. 2000; Millar et al. 2003], plastids and cytosol for glutathione [Wachter et al. 2005]) to the other cell compartments as they are used for Cd and ROS detoxification in the cytosol. This initial phase is then followed by a strong accumulation of glutathione and ascorbate (Fig. 7) which indicates an acclimation or resistance phase where the plant is successfully coping with the environmental stress condition (Tausz et al. 2004; Kranner et al. 2010). Nevertheless, wildtype plants exposed to 100 μM Cd obviously could not withstand Cd stress successfully in the long term as symptoms such as chlorosis and necrotic spots across the leaves could be observed 14 days after Cd exposure. These symptoms could be correlated with a strong decrease in glutathione contents in most cell compartments at this time point. Additionally, ascorbate contents remained at or below control levels in most cell compartments (Fig. 7) indicating that these plants went through an exhaustion phase which is characterized by failure of protection and repair mechanisms (Kranner et al. 2010) and led to strong symptoms such as the appearance of chlorosis and necrotic spots.
Line drawing showing a response curve of the overall ascorbate and glutathione labeling of Col-0, pad2-1, and vtc2-1 to treatment with different concentrations of Cd. The relative ascorbate and glutathione content was obtained according to Koffler et al. (2013) using the corresponding labeling density during Cd treatment and the relative compartment volume from the leaf center, where the sum runs over all compartments. The alarming phase is indicated with the letter "A", the resistance phase is indicated with the letter "R", and the exhaustion phase is indicated with the letter "E". Black line glutathione, grey line ascorbate, asterisk appearance of symptoms
Glutathione contents in pad2-1 mutants which showed highest sensitivity to Cd were in general decreased in all cell compartments throughout the treatment with 50 and 100 μM Cd (Fig. 7). This strong decrease was correlated with strong symptom development early after Cd treatment (e.g., 96 h after treatment with 100 μM Cd) indicating that low glutathione levels in this mutant which contains up to 90 % less glutathione than wildtype plants (Parisy et al. 2006; Zechmann et al. 2008) were unable to protect plants against the toxic effects of Cd (Fig. 7). Similar effects have also been found in plants with artificially decreased glutathione contents and for the glutathione deficient mutant cad2-1 which showed higher Cd sensitivity correlated with low glutathione contents (Howden et al. 1995; Wójcik and Tukiendorf 2011). Considering that pad2-1 mutants showed the highest sensitivity to Cd, it becomes obvious that the accumulation of ascorbate in pad2-1 mutants treated with 50 μM Cd were not able to compensate low glutathione contents in order to prevent symptom development induced by the accumulation of ROS commonly observed during Cd treatment (Gill and Tuteja 2010; Yadav 2010). On the subcellular level, it is interesting that higher Cd sensitivity correlated with a strong reduction of glutathione in mitochondria. The pad2-1 mutant is characterized by low glutathione contents in all cell compartments (up to 90 % less than Col-0) and wildtype glutathione levels in mitochondria. Additionally, we have recently demonstrated that high and stable levels of glutathione in mitochondria of the pad2-1 mutants are important for proper plant development (Zechmann and Müller 2010). Thus, the strong decrease of glutathione contents in mitochondria observed in this study of up to 90 % in this mutant could be a key factor for higher Cd sensitivity.
vtc2-1 mutants reacted to treatment of 50 μM Cd with a bell-shaped stress–response curve (Kranner et al. 2010) in most cell compartments with an initial decrease of glutathione and ascorbate followed by a strong accumulation of both antioxidants and a final drop to or below control values (Fig. 7). vtc2-1 mutants treated with 100 μM Cd showed a strong decrease of glutathione contents similar to the pad2-1 mutants but reacted with a strong increase of ascorbate contents after 48 days. Nevertheless, similar to the pad2-1 mutants, a strong decrease of both antioxidants could be observed in the long term at 100 μM Cd treatment which correlates well with symptom development such as chlorosis and necrosis on the leaves. In this respect it is remarkable that the vtc2-1 mutants which have impaired ascorbate synthesis leading to 60 % less ascorbate in the tissue when compared to Col-0 (Conklin et al. 2000; Zechmann et al. 2011c) reacted with a strong increase in ascorbate contents upon Cd treatment (observed mainly at 48 and 96 h after treatment). Similar results have also been observed during exposure to high light where vtc2-1 mutants surprisingly reacted with an increase in ascorbate contents despite distorted ascorbate synthesis (Müller-Moulé et al. 2004).
On the subcellular level, it is interesting that gold particles bound to glutathione could not be detected in vacuoles even in Cd-treated samples. It is commonly accepted that glutathione forms complexes with Cd through the thiol groups which are then sequestered in vacuoles (Rauser 2001; Maksymiec and Krupa 2006; Semane et al. 2007; DalCorso et al. 2008; Jozefczak et al. 2012). Thus, the lack of labeling in vacuoles in Cd-treated samples indicates that the glutathione antibody method used in this study detects free glutathione only. These results are supported by previous labeling experiments which showed the lack of labeling in vacuoles of Arabidopsis leaf disks treated with monochlorobimane which are similar to Cd bound to the thiol groups of reduced glutathione and then transported into vacuoles (Queval et al. 2011). Long-term exposure to Cd (14 days) induced the depletion of glutathione and ascorbate contents in all cell compartments except chloroplasts. These results indicate an important role for antioxidants in chloroplasts for the protection of plants against Cd. It is well known that Cd inhibits photosynthesis as it disturbs the synthesis of chlorophyll and carotenoids, inhibits the enzyme activity of the Calvin cycle and leads to CO2 deficiency due to stomatal closure, which cause the inhibition of the photosynthesis (Ding et al. 2006). As glutathione and ascorbate decreased in all other cell compartments, these results indicate that the plant accumulated antioxidants in chloroplasts at the expense of ascorbate and glutathione of the other cell compartments. This is especially interesting as the cytosol is supposed to be the primary cell compartment for Cd detoxification as it is not distributed to other cell compartments except vacuoles (Van Belleghem et al. 2007). Thus, the long-term accumulation of ascorbate and glutathione in chloroplasts indicates that the plant redistributes glutathione and ascorbate into chloroplasts during long-term Cd exposure most probably to detoxify ROS produced during photosynthesis.
Conclusion
Summing up, we can conclude that ascorbate and glutathione deficient mutants showed higher sensitivity to Cd. Nevertheless, glutathione deficient pad2-1 mutants showed even higher sensitivity to Cd and reacted to Cd with a strong decrease in glutathione contents similar to vtc2-1 mutants. As symptom development seemed to be related with low glutathione contents rather than low ascorbate contents (Fig. 7), it seems that low glutathione contents rather than low ascorbate contents are responsible for the high Cd sensitivity in these plants.
References
Akhter F, McGarvey B, Macfie SM (2012) Reduced translocation of cadmium from roots is associated with increased production of phytochelatins and their precursors. J Plant Physiol 169:1821–1829
Anjum NA, Umar S, Ahmad A et al (2008) Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul 54:271–279
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate–glutathione cycle and glutathione metabolism. Plant Physio Biochem 43:107–116
Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123:335–343
Baryla A, Carrier P, Franck F et al (2001) Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth. Planta 212:696–709
Bouzon ZL, Ferreira EC, dos Santos R, Scherner F, Horta PA, Maraschin M, Schmidt EC (2012) Influences of cadmium on fine structure and metabolism of Hypnea musciformis (Rhodophyta, Gigartinales) cultivated in vitro. Protoplasma 249:637–650
Van Belleghem F, Cuypers A, Semane B et al (2007) Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol 173:495–508
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Cairns NG, Pasternak M, Wachter A et al (2006) Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo 1[C]. Plant Physiol 141:446–455
Chao Y, Hong C, Chen C, Kao CH (2011) The importance of glutathione in defence against cadmium-induced toxicity of rice seedlings. Crop, Environ Bioinforma 8:217–228
Chen YX, He YF, Luo YM et al (2003) Physiological mechanism of plant roots exposed to cadmium. Chemosphere 50:789–793
Clemens S, Schroeder JI, Degenkolb T (2001) Caenorhabdites elegans expresses a functional phytochelatin synthase. Eur J Biochem 268:3640–3643
Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154:847–856
DalCorso G, Farinati S, Furini A (2010) Regulatory networks of cadmium stress in plants. Plant Signal Behav 5:663–667
DalCorso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50:1268–1280
Ding B, Shi G, Xu Y et al (2006) Physiological responses of Alternanthera philoxeroides (Mart.) Griseb leaves to cadmium stress. Environ Pollut 147:800–803
Ekmekci Y, Tanyolac D, Ayhan B (2008) Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J Plant Physiol 165:600–611
Fernandez-Garcia N, Marti MC, Jimenez A, Sevilla F, Olmos E (2009) Sub-cellular distribution of glutathione in an Arabidopsis mutant (vtc1) deficient in ascorbate. J Plant Physiol 166:2004–2012
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Gallego SM, Pena LB, Barcia RA et al (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–9030
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Review: making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Gratão PL, Monteiro CC, Antunes AM et al (2008) Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann Appl Biol 153:321–333
Gratão PL, Monteiro CC, Rossi ML et al (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ Exp Bot 67:387–394
Gratão PL, Monteirob CC, Carvalhoa RF et al (2012) Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiology and Biochemistry 56:79–96
He J-Y, Ren Y-F, Zhu C et al (2008) Effect of Cd on growth, photosynthetic gas exchange, and chlorophyll fluorescence of wild and Cd-sensitive mutant rice. Photosynthetica 46:466–470
Hegedüs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093
Howden R, Andersen CR, Coldsbrough B, Cobbett CS (1995) A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 107:1067–1073
Iannone MF, Rosales EP, Groppa MD, Benavides MP (2010) Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma 245:15–27
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Keunen E, Remans T, Opdenakker K et al (2013) A mutant of the Arabidopsis thaliana LIPOXYGENASE1 gene shows altered signalling and oxidative stress related responses after cadmium exposure. Plant Physiol Biochem 63:272–280
Koffler BE, Bloem E, Zellnig G, Zechmann B (2013) High resolution imaging of subcellular glutathione concentrations by quantitative immunoelectron microscopy in different leaf areas of Arabidopsis. Micron 45:119–128
Kolb D, Müller M, Zellnig G, Zechmann B (2010) Cadmium induced changes in subcellular glutathione contents within glandular trichomes of Cucurbita pepo L. Protoplasma 243:87–94
Kranner I, Minibayeva FV, Beckett RP, Seal CE (2010) What is stress? Concepts, definitions and applications in seed science. New Phytol 188:655–673
Liu Y, Wang X, Zeng G et al (2007) Cadmium-induced oxidative stress and response of the ascorbate-glutathione cycle in Bechmeria nivea (L.) Gaud. Chemosphere 69:99–107
Maksymiec W, Krupa Z (2006) The effects of short-term exposition to Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ Exp Bot 57:187–194
Maughan SC, Pasternak M, Cairns N et al (2010) Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. PNAS 107:2331–2336
Mendoza-cózatl DG, Butko E, Springer F et al (2008) Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J 54:249–259
Millar AH, Mittova V, Kiddle G et al (2003) Control of ascorbate aynthesis by respiration and its implications for stress responses. Plant Physiol 133:443–447
Mohamed AA, Castagna A, Ranieri A, Sanità di Toppi L (2012) Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol Biochem 57:15–22
Monteiro CC, Carvalho RF, Gratão PL et al (2011) Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ Exp Bot 71:306–320
Müller-Moulé P, Golan T, Niyogi KK (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol 134:1163–1172
Noctor G, Mhamdi A, Chaouch S et al (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Noriega G, Caggiano E, Lecube ML et al (2012) The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 25:1155–1165
Paradiso A, Berardino R, De Pinto MC et al (2008) Increase in ascorbate–glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374
Parisy V, Poinssot B, Owsianowski L et al (2006) Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J 49:159–172
Queval G, Jaillard D, Zechmann B, Noctor G (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ 34:21–32
Rauser WE (2001) The role of glutathione in plant reaction and adaptation to excess metals. In: Grill D, Tausz M, Cok LJ (eds) Significance of glutathione to plant adaptation to the environment. Kluwer, Dordrecht, pp 123–154
Sanità di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Sanità di Toppi L, Pawlik-Skowrońska B, Vurro E et al (2008) First and second line mechanisms of cadmium detoxification in the lichen photobiont Trebouxia impressa (Chlorophyta). Environ Pollut 151:280–286
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Semane B, Cuypers A, Smeets K et al (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plantarum 129:519–528
Shan S, Liu F, Chunjuan Li C, Wan S (2012) Effects of cadmium on growth, oxidative stress and antioxidant enzyme activities in peanut (Arachis hypogaea L.) seedlings. J Agricult Sci 4:142–151
Smeets K, Opdenakker K, Remans T et al (2009) Oxidative stress-related responses at transcriptional and enzymatic levels after exposure to Cd or Cu in a multipollution context. J Plant Physiol 166:1982–1992
Smiri M, Chaoui A, Rouhier N et al (2011) Cadmium affects the glutathione/glutaredoxin system in germinating pea seeds. Biol Trace Elem Res 142:93–105
Tausz M, Sircelj H, Grill D (2004) The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J Exp Bot 55:1955–1962
Vernoux T, Wilson RC, Ka S et al (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12:97–110
Wachter A, Wolf S, Steininger H et al (2005) Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 41:15–30
Wang Y, Zong K, Jiang L et al (2011) Characterization of an Arabidopsis cadmium-resistant mutant cdr3-1D reveals a link between heavy metal resistance as well as seed development and flowering. Planta 233:697–706
Wójcik M, Tukiendorf A (2011) Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biol Plantarum 55:125–132
Xu P, Zou J, Meng Q et al (2008) Effects of Cd(2+) on seedling growth of garlic (Allium sativum L.) and selected physiological and biochemical characters. Bioresource Technol 99:6372–6378
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Zechmann B, Koffler BE, Russell SD (2011a) Glutathione synthesis is essential for pollen germination in vitro. BMC 11:54
Zechmann B, Liou L-C, Koffler BE et al (2011b) Subcellular distribution of glutathione and its dynamic changes under oxidative stress in the yeast Saccharomyces cerevisiae. FEMS 11:631–42
Zechmann B, Stumpe M, Mauch F (2011c) Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 233:1–12
Zechmann B, Mauch F, Sticher L, Müller M (2008) Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids. J Exp Bot 59:4017–4027
Zechmann B, Müller M (2010) Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 246:15–24
Zechmann B, Müller M (2008) Effects of zucchini yellow mosaic virus infection on the subcellular distribution of glutathione and its precursors in a highly tolerant Cucurbita pepo cultivar. Botany 86:1092–1100
Zelinova V, Mistrik I, Pavlovkin J, Tamas L (2013) Glutathione peroxidase expression and activity in barley root tip after short-term treatment with cadmium, hydrogen peroxide and t-butyl hydroperoxide. Protoplasma. doi:10.1007/s00709-013-0481-3
Acknowledgement
This work was supported by the Austrian Science Fund (FWF 22988-B16).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig.S1
Representative transmission electron micrographs showing gold particles bound to ascorbate on leaf sections from Arabidopsis thaliana Col-0. Plants were treated with 0 (a), 50 (b–d), and 100 μM Cd (e–g) for 12 h (b, e), 48 h (c, f), and 14 days (a, d, g). Bars = 0.5 μm. C chloroplasts with or without starch (St), CW cell walls, M mitochondria, N nuclei, Px peroxisomes, V vacuoles (JPEG 5313 kb)
Fig. S2
Representative transmission electron micrographs showing gold particles bound to ascorbate on leaf sections from Arabidopsis thaliana pad2-1. Plants were treated with 0 (a), 50 (b–d), and 100 μM Cd (e–g) for 12 h (b, e), 96 h (c, f), and 14 days (a, d, g). Bars = 0.5 μm. C chloroplasts with or without starch (St), CW cell walls, M mitochondria, N nuclei, Px peroxisomes, V vacuoles (JPEG 5448 kb)
Fig. S3
Representative transmission electron micrographs showing gold particles bound to ascorbate on leaf sections from Arabidopsis thaliana vtc2-1. Plants were treated with 0 (a), 50 (b–d), and 100 μM Cd (e–g) for 12 h (b, e), 48 h (c, f), and 14 days (a, d, g). Bars = 0.5 μm. C chloroplasts with or without starch (St), CW cell walls, M mitochondria, N nuclei, Px peroxisomes, V vacuoles (JPEG 5514 kb)
Fig. S4
Representative transmission electron micrographs showing gold particles bound to glutathione on leaf sections from Arabidopsis thaliana Col-0. Plants were treated with 0 (a), 50 (b–d), and 100 μM Cd (e–g) for 12 h (b, e), 48 h (c, f), and 14 days (a, d, g). Bars = 0.5 μm. C chloroplasts with or without starch (St), CW cell walls, M mitochondria, N nuclei, Px peroxisomes, V vacuoles (JPEG 5347 kb)
Fig. S5
Representative transmission electron micrographs showing gold particles bound to glutathione on leaf sections from Arabidopsis thaliana pad2-1. Plants were treated with 0 (a), 50 (b–d), and 100 μM Cd (e–g) for 12 h (b, e), 96 h (c, f), and 14 days (a, d, g). Bars = 0.5 μm. C chloroplasts with or without starch (St), CW cell walls, M mitochondria, N nuclei, Px peroxisomes, V vacuoles (JPEG 5448 kb)
Fig. S6
Representative transmission electron micrographs showing gold particles bound to glutathione on leaf sections from Arabidopsis thaliana vtc2-1. Plants were treated with 0 (a), 50 (b–d), and 100 μM Cd (e–g) for 12 h (b, e), 48 h (c, f), and 14 days (a, d, g). Bars = 0.5 μm. C chloroplasts with or without starch (St), CW cell walls, M mitochondria, N nuclei, Px peroxisomes, V vacuoles (JPEG 5246 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Koffler, B.E., Polanschütz, L. & Zechmann, B. Higher sensitivity of pad2-1 and vtc2-1 mutants to cadmium is related to lower subcellular glutathione rather than ascorbate contents. Protoplasma 251, 755–769 (2014). https://doi.org/10.1007/s00709-013-0576-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0576-x