Summary.
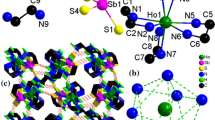
The reaction of elemental antimony with elemental sulfur and [Ph4P]Br in an aqueous solution of neopentanediamine under solvothermal conditions yields yellow needles of the new thioantimonate(III) [Ph4P]2[Sb6S10]. The structure consists of [Ph4P]+ cations and infinite one-dimensional anionic (Sb6S10 2−)n chains running along the crystallographic a axis. The chains are composed of 10-membered Sb5S5 rings with alternating Sb and S atoms and separated by the tetraphenylphosphonium cations. Upon heating the compound decomposes in two distinct steps, starting at about 100°C. The final product was identified by X-ray powder diffractometry as Sb2S3.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received December 17, 1999. Accepted (revised) February 7, 2000
Rights and permissions
About this article
Cite this article
Rijnberk, H., Näther, C. & Bensch, W. Solvothermal Synthesis, Crystal Structure Determination, and Properties of the Thioantimonate [Ph4P]2[Sb6S10]. Monatshefte fuer Chemie 131, 721–726 (2000). https://doi.org/10.1007/s007060070072
Issue Date:
DOI: https://doi.org/10.1007/s007060070072