Abstract
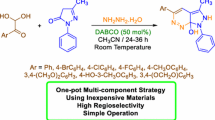
The new type of chemical one-pot Knoevenagel-Michael reaction with following stereoselective NBS induced cyclization was found: the direct chemical one-pot transformation of aldehydes and two molecules of pyrazolin-5-one into substituted bispyrazolone cyclopropanes in 85–95% yields. This stereoselective one-pot process is very efficient and convenient way to substituted (R*,R*)-bis(spiro-2,4-dihydro-3H-pyrazol-3-one)cyclopropanes – useful compounds for different biomedical applications, using reasonable and non-expansive starting materials. Mild and facile conditions of this chemical cascade one-pot process and simple reasonable non-chromatographic isolation procedure allow excellent substance yields along with superior stereoselectivity.
Graphical abstract

Similar content being viewed by others
Data availability
1H and 13C NMR spectra for all new compounds are presented in in the supplementary information file.
References
Brahmachar G (2016) RSC Adv 6:64676
Baruaha B, Deb ML (2021) Org Biomol Chem 19:1191
Lu L-Q, Chen J-R, Xiao W-J (2012) Acc Chem Res 45:1278
Xu P-F, Wang W (eds) (2014). John Wiley and Sons, Hoboken
Faust R (2001) Angew Chem Int Ed 40:2251
Talele TT (2016) J Med Chem 59:8712
Časar Z (2020) Synthesis 52:1315
Laroche C, Behr J-B, Szymoniak J, Bertus P, Schutz C, Vogel P, Plantier-Royon R (2006) Bioorg Med Chem 14:4047
Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Wu T, He Y (2006) Bioorg Med Chem Lett 16:2105
Schmidt A, Dreger A (2011) Curr Org Chem 15:1423
Elguero J, Goya P, Jagerovic N, Silva AMS (2002) Pyrazoles as Drugs. Italian Society of Chemistry, Rome
Kucukguzel SG, Senkardes S (2015) Eur J Med Chem 97:786
Lapchak PA (2010) Expert Opin Pharmacother 11:1753
Montijo-Barrios E, Cadena F, Ramírez-Mayans JA, Pedro Gutiérrez-Castrellón P (2011) Rev Invest Clin 63:335
Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, Arning M, Provan D, Jenkins JM (2007) N Engl J Med 357:2237
Miljković MN, Rančić NK, Simić RM, Stamenković DM, Dragojević-Simić VM (2018) Hosp Pharm 5:694
Gouda MA, Al-Balawi MMM, Abu-Hashem AA (2016) Eur J Chem 7:363
Sujatha K, Shanthi G, Selvam NP, Manoharan S, Perumal PT, Rajendran M (2009) Bioorg Med Chem Lett 19:4501
Sugiura S, Ohno S, Ohtani O, Izumi K, Kitamikado T, Asai H, Kato K (1977) J Med Chem 20:80
Diana P, Carbone A, Barraja P, Martorana A, Gia O, DallaVia L, Cirrincione G (2007) Bioorg Med Chem Lett 17:6134
Cadena-Cruz JE, Guamán-Ortiz LM, Romero-Benavides JC, Bailon-Moscoso N, Murillo-Sotomayor KE, Ortiz-Guamán NV, Heredia-Moya J (2021) BMC Chem 15:N38
Bailey DM, Hansen PE, Hlavac AG, Baizman ER, Pearl J, Defelice AF, Feigenson ME (1985) J Med Chem 28:256
Mahajan RN, Havaldar FH, Fernandes PS (1991) J Indian Chem Soc 68:245
Chauhan PMS, Singh S, Chatterjee RK (1993) Indian J Chem Sect B: Org Chem Incl. Med Chem 32:858
Pettinari C, Marchetti F, Pettinari R, Drozdov A, Troyanov S, Voloshin AI, Shavaleen NM (2002) J Chem Soc Dalton Trans 2002:1409
Moegling J, Benischke AD, Hammann JM, Vepřek NA, Zoller F, Rendenbach B, Hoffmann A, Sievers H, Schuster M, Knochel P, Herres-Pawlis S (2015) Eur J Org Chem 2015:7475
Devi S, Nayak A, Mittra AS (1984) J Indian Chem Soc 61:640
Itokawa M, Miyata T, Arai M (2010) Detection and Treatment of Schizophrenia. Eur Pat EP 2189537, May 26, 2010; (2013) Chem Abstr 159:467277
Itokawa M, Miyata T, Arai M (2013) Examination and treatment of schizophrenia. Patent JP 5288365, Sep 11, 2013; (2013) Chem Abstr 159:467277
Mittra AS, Rout MK (1969) J Indian Chem Soc 46:890
Westoo G (1957) Acta Chem Scand 11:1359
Elinson MN, Vereshchagin AN, Tretyakova EO, Bushmarinov IS, Nikishin GI (2011) Synthesis 2011:3015
Barreiro-Costa O, Morales-Noboa G, Rojas-Silva P, Lara-Barba E, Santamaría-Aguirre J, Bailón-Moscoso N, Romero-Benavides JC, Ana Herrera A, Cueva C, Ron-Garrido L, Poveda A, Heredia-Moya J (2021) Molecules 26:4960
Elinson MN, Dorofeeva EO, Vereshchagin AN, Nasybullin RF, Egorov MP (2013) Cat Sci Technol 5:2384
Elinson MN, Ryzhkova YE, Vereshchagin AN, Ryzhkov FV, Kalashnikova VM, Egorov MP (2021) Monatsh Chem 152:641
Elinson MN, Dorofeeva EO, Vereshchagin AN, Korshunov AD, Egorov MP (2016) Res Chem Intermed 42:2191
Elinson MN, Vereshchagin AN, Ryzhkov FV (2016) Chem Rec 16:1950
Vereshchagin AN, Elinson MN, Dorofeeva EO, Zaimovskaya TA, Stepanov NO, Gorbunov SV, Belyakov PA, Nikishin GI (2012) Tetrahedron 68:1198
Elinson MN, Vereshchagin AN, Stepanov NO, Belyakov PA, Nikishin GI (2010) Tetrahedron Lett 51:6598
Elinson MN, Feducovich SK, Stepanov NO, Vereshchagin AN, Nikishin GI (2008) Tetrahedron 64:708
Vereshchagin AN, Elinson MN, Stepanov NO, Nikishin GI (2009) Mendeleev Commun 19:324
Elinson MN, Vereshchagin AN, Stepanov NO, Ilovaisky AI, Vorontsov AY, Nikishin GI (2009) Tetrahedron 65:6057
Elinson MN, Vereshchagin AN, Stepanov NO, Zaimovskaya TA, Merkulova VM, Nikishin GI (2010) Tetrahedron Lett 51:428
Vereshchagin AN, Elinson MN, Zaimovskaya TA, Nikishin GI (2013) Tetrahedron 69:1945
Rosen T (1991) The Perkin Reaction. In: Trost BM, Flemingand I, Heathcock CH (eds) Comprehensive Organic Synthesis, vol 2. Pergamon, Oxford, p 395
Elinson MN, Feducovich SK, Zaimovskaya TA, Vereshchagin AN, Nikishin GI (2005) Russ Chem Bull 54:673
Cleary T, Rawalpally T, Kennedy N, Chaves A (2010) Tetrahedron Lett 45:1533
Elinson MN, Vereshchagin AN, Feducovich SK, Zaimovskaya TA, Starikova ZA, Belyakov PA, Nikishin GI (2007) Tetrahedron Lett 48:6614
Saikia I, Borah AJ, Phukan P (2016) Chem Rev 116:6837
Wang JY, Zhou P, Li G, Hao WJ, Tu SJ, Jiang B (2017) Org Lett 19:6682
Wei Y, Lin S, Xue H, Liang F, Zhao B (2012) Org Lett 14:712
Huang C, Zeng Y, Cheng H, Hu A, Liu L, Xiao Y, Zhang J (2017) Org Lett 19:4968
Chakrabarty M, Kundu T, Arima S, Harigaya Y (2005) Tetrahedron Lett 46:2865
Nikishin GI, Elinson MN, Lizunova TL (1991) Tetrahedron Lett 32:2655
Vereshchagin AN, Elinson MN, Zaimovskaya TA, Nikishin GI (2008) Tetrahedron 64:9766
Elinson MN, Dorofeeva EO, Vereshchagin AN, Nikishin GI (2015) Russ Chem Rev 84:485
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elinson, M.N., Ryzhkova, Y.E., Ryzhkov, F.V. et al. Stereoselective and efficient chemical transformation of aldehydes and two molecules of pyrazolin-5-one into bis(spiropyrazolone)cyclopropanes under a column chromatography-free protocol at room temperature. Monatsh Chem 154, 625–633 (2023). https://doi.org/10.1007/s00706-023-03072-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03072-5