Abstract
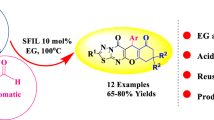
Herein, a novel series of chromenopyrido[3,2-e]isothiazolo[2,3-a]pyrimidines were synthesized by a three-component reaction of 4-hydroxy coumarin, aromatic aldehydes, and 5-amino-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-7-one under catalyst-free conditions in PEG 400 as a green solvent. The optimal reaction condition of this reaction was determined. The obtained product’s structure was elucidated via NMR, IR, mass spectra, and elemental analysis techniques. This protocol has various advantages: excellent yields, short reaction times, no column chromatography, and green reaction media.
Graphical abstract





Similar content being viewed by others
References
A. Hasaninejad, A. Zare, M. Shekouhy, Green Chem. 7, 64 (2005)
D. Fang, J. Luo, X.L. Zhou et al., Catal. Lett. 116, 76 (2007)
T. Itoh, K. Nishimura, K. Nagata et al., Synth. Lett. 14, 2207 (2006)
J.W. Yang, M. Stadler, B. List, Angew. Chem. Int. Ed. 46, 609 (2007)
A. Hasaninejad, A. Zare, M. Shekouhy, N. Golzar, Org. Prep. Proced. Int. 43, 131 (2011)
M. Cortes-Clerget, J. Yu, J.R. Kincaid, P. Walde, F. Gallou, B.H. Lipshutz, Chem. Sci. 12, 4237 (2021)
H. Pang, Y. Hu, J. Yu, F. Gallou, B.H. Lipshutz, J. Am. Chem. Soc. 143, 3373 (2021)
G.Y. Guo, T. Yang, H.X. Gao, Green Chem. 6, 75 (2004)
M. Kidwai, D. Bhatnagar, N.K. Mishra, V. Bansal, Catal. Commun. 9, 2547 (2008)
X.C. Wang, H.P. Gong, Z.J. Quan, L. Li, H.L. Ye, Chin. Chem. Lett. 20, 44 (2009)
R. Mohebat, A.Y.E. Abadi, M.T. Maghsoodlou, M. Mohammadi, Res. Chem. Intermed 42, 5915 (2016)
D.J. Heldebrant, P.G. Jessop, J. Am. Chem. Soc. 125, 5600 (2003)
C. Ding, S. Li, K. Feng, M. Chen, Green Chem. (2021).
H.A. Younus, M. Al-Rashida, A. Hameed, M. Uroos, U. Salar, S. Rana, K.M. Khan, Expert Opin. Ther. Pat. 31, 267 (2021)
S. E. John, S. Gulati, N. Shankaraiah, Organic Chemistry Frontiers. (2021)
M.M. Khan, B. Saigal, S. Shareef, S. Khan, S.C. Sahoo, Synth. Commun. 48, 2683 (2018)
M.M. Khan, S.S. Khan, J Heterocycl. Chem. 56, 1020 (2019)
B. Saigal, S. Khan, H. Rahman, M.M. Khan, RSC Adv. 9, 14477 (2019)
M.M. Khan, S. Khan, S. Saigal, A. Singh, Tetrahedron Lett 60, 150996 (2019)
V. Sharma, M. Gupta, P. Kumar, A. Sharma, Curr. Pharm. Des. 27, 15 (2021)
N. Bhardwaj, A. Pathania, P. Kumar, Curr. Tradit. Med. 7, 5 (2021)
G. Yadav, S. Ganguly, Eur. J. Med. Chem. 97, 419 (2015)
D. Choudhary, R. Birle, N. Kayande, S. Patil, Int. J. Res. Eng. Sci. Manag. 4, 131 (2021)
P.J. Borpatra, B. Deka, M.L. Deb, P.K. Baruah, Org. Chem. Front. 6, 3445 (2019)
J.A. Bull, J.J. Mousseau, G. Pelletier, A.B. Charette, Chem. Rev. 112, 2642 (2012)
J.-P. Wan, Y. Liu, RSC Adv. 2, 9763 (2012)
R. Boer, V. Gekeler, Drugs of the Future. 20, 499 (1995)
R. Shan, C. Velazquez, E. Knaus, Med. Chem. 47, 254 (2004)
T. Godfaid, R. Miller, M. Wibo, Pharmacol. Rev. 38, 321 (1986)
A. Sausins, G. Duburs, Heterocycles 27, 279 (1988)
P.P. Mager, R.A. Coburn, A.J. Solo, D.J. Trigle, H. Rothe, Drug Des. Discovery 8, 273 (1992)
R. Mannhold, B. Jablonka, W. Voigdt, K. Schoenafinger, K. Schravan, Eur. J. Med. Chem. 27, 229 (1992)
R. Peri, S. Padmanabhan, A. Rutledge, S. Singh, D.J. Triggle, J. Med. Chem. 43, 2906 (2000)
R. Alajarin, J.J. Vaquero, J. Alvarez-Builla, M. Pastor, C. Sunkel, M. Fau de Casa-Juana, J. Priego, P.R. Statkow, J. Sanz-Aparicio, I. Fonseca, J. Med. Chem. 38, 2830 (1995)
F.R. Buhler, W. Kiowski, J. Hypertens. 5, S3 (1987)
J.L. Reid, P.A. Meredith, F. Pasanisi, J. Cardiovasc. Pharmacol. 7, S18 (1985)
M.S. Frasinyuk, S.V. Gorelov, S.P. Bondarenko, V.P. Khilya, Chem, Heterocycl. Compd. 45, 1261 (2009)
Z. Chen, J. Bi, W. Su, Chin. J. Chem. 31, 507 (2013)
R. Miri, R. Motamedi, M.R. Rezaei, O. Firuzi, A. Javidnia, A. Shafiee, Arch. Pharm. Chem. Life Sci. 2, 111 (2011)
D. Srikrishna, C. Godugu, P.K. Dubey, Mini Rev. Med. Chem. 18, 113 (2018)
F. Borges, F. Roleira, N. Milhazes, L. Santana, E. Uriarte, Curr. Med. Chem. 12, 887 (2005)
K. Bhagat, J. Bhagat, M.K. Gupta, J.V. Singh, H.K. Gulati, A. Singh, K. Kaur, G. Kaur, S. Sharma, A. Rana, H. Singh, ACS Omega 4, 8720 (2019)
S.A. Azim, S.M. Al-Hazmy, E.M. Ebeid, S.A. El-Daly, Opt. Laser Technol. 37, 245 (2005)
G. Srinivasan, J. Chen, J. Parisi, C. Brückner, X. Yao, Y. Lei, Appl. Biochem. Biotechnol. 177, 1115 (2015)
E.E. Flefel, M.A. Salama, M. El-Shahat, Phosphorus. Sulfur Silicon Relat. Elem. 182, 1739 (2007)
A.A. Abu-Hashem, M.M. Youssef, H.A.R. Hussein, J. Chin. Chem. Soc. 58, 41 (2011)
B. Tozkoparan, M. Ertan, P. Kelicen, R. Demirdamar, II Farmaco. 54, 588 (1999)
O. Alam, S.A. Khan, N. Siddiqui, W. Ahsan, Med. Chem. Res. 19, 1245 (2010)
S.F. Mohamed, E.M. Flefel, A.E. Amra, D.N. Abd El-Shafy, Eur. J. Med. Chem. 45, 1494 (2010)
R.A. Azzam, R.R. Osman, G.H. Elgemeie, ACS Omega 5, 1640 (2020)
B. Pan, R. Huang, L. Zheng, C. Chen, S. Han, D. Qu, M. Zhu, P. Wei, Eur. J. Med. Chem. 46, 819 (2011)
S.V. Gupta, K.G. Baheti, S.B. Ganorkar, D. Dekhane, S. Pawar, S.N. Thore, Med. Chem. Res. 22, 1065 (2013)
M. Ashok, B.S. Holla, N.S. Kumari, Eur. J. Med. Chem. 42, 380 (2007)
M.L. Deb, P.J. Borpatra, P.K. Baruah, Green Chem. 21, 69 (2019)
P. Patra, G.K. Kar, New J Chem. 45, 2879 (2021)
G.M. Conlin, J.R. Gear, J. Nat. Prod. 56, 1402 (1993)
R. Miri, R. Motamedi, M.R. Rezaei, O. Firuzi, A. Javidnia, A. Shafiee, Arch Pharm Chem Life Sci 2, 111 (2011)
P. Patra, Chemistry Select 4, 2024 (2019)
K.V. Sashidhara, G.R. Palnati, L.R. Singh, A. Upadhyay, S.R. Avula, A. Kumar, R. Kant, Green Chem. 17, 3766 (2015)
W. Stadlbauer, Monatsh. Chem. 118, 1297 (1987)
A.T. Khan, D.K. Das, K. Islam, P. Das, Tetrahedron Lett. 53, 6418 (2012)
A.T. Khan, D.K. Das, Tetrahedron Lett. 53, 2345 (2012)
Z. Chen, L. Hu, F. Peng, Synlett 12, 1888 (2016)
M. Adib, F. Peytam, M. Rahmanian-Jazi, M. Mohammadi-Khanaposhtani, S. Mahernia, H.R. Bijanzadeh, M. Jahani, S. Imanparast, M.A. Faramarzi, M. Mahdavi, B. Larijani, New J. Chem. 42, 17268 (2018)
S. Pal, M.N. Khan, S. Karamthulla, L.H. Choudhury, RSC Adv. 3, 15705 (2013)
D. Wang, D.-L. Wang, X.-C. Shi, J.-H. Qian, Heterocycles 92, 2141 (2016)
T. Tabibi, A.A. Esmaeili, J.T. Mague, Mol. Diversity 3, 1 (2021)
M. Zangouei, A.A. Esmaeili, A. Mague, J. T. Tetrahedron. 73, 2894 (2017)
M. Zangouei, A.A. Esmaeili, J.T. Mague, Synlett 27, 1669 (2016)
S. Jannati, A.A. Esmaeili, Tetrahedron 74, 2967 (2018)
M. Esmaeilinezhad, A.A. Esmaeili, S. Jannati, J. Chem. Res. 42, 618 (2018)
M. Zangouei, A.A. Esmaeili, A. Habibi, A.R. Fakhari, Tetrahedron 70, 8619 (2014)
H. Furrer, E. Granzer, R. Wagner, Eur. J. Med. Chem. 29, 819 (1994)
Acknowledgements
The Research Council of the Ferdowsi University of Mashhad is acknowledged for the financial support (Grant No. 3/50328).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Danehchin, M., Esmaeili, A.A. Efficient synthesis of novel chromenopyrido[3,2-e]isothiazolo[2,3-a]pyrimidines via a non-catalytic one-pot three-component reaction. Res Chem Intermed 48, 629–646 (2022). https://doi.org/10.1007/s11164-021-04613-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04613-5