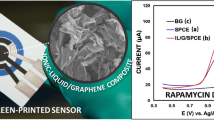
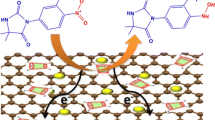
Abstract
Dasatinib is an anticancer drug that treats acute lymphoblastic leukemia, chronic myelogenous leukemia, and prostate cancer with several side effects. In this research, we suggest nanoparticle-modified screen-printed electrodes (SPCEs) as disposable electrochemical sensors for fast quantification of dasatinib in pharmaceutical formulations. Carbon nanotubes, single-walled carbon nanotubes (SWCNT), graphene, and graphene oxide-modified SPCEs were characterized by scanning electron microscopy. The study also recommends SWCNT-modified SPCEs as the best-performing electrode for determining dasatinib, demonstrating an excellent boosting effect on the oxidation response of dasatinib. This was accomplished using the square-wave voltammetry method. After optimization of the pH condition, pH 5.0 Britton–Robinson buffer, SWCNT-modified SPCEs demonstrated 94% recovery with optimum electro-oxidation activity. The oxidation currents exhibited linear relation with dasatinib concentration in the 0.1–100 µM. Based on the results, a limit of detection of 0.06 µM was obtained in the standard solution. The SWCNT-modified SPCEs have been applied to analyze dasatinib in pharmaceutical tablet samples. The demonstrated performance beats all comparable standard analytical tools and presumably may be used for general drug quantitation in pharmaceutical tablets.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dasatinib (DAS) or N-(2-chloro-6-methylphenyl)-2-[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-ylamino]-thiazole-5-carboxamide monohydrate (Fig. S1) is an orally accessible multitargeted inhibitor to kinases of the SRC family (sarcoma), PDGFR-β5 (platelet-derived growth factor receptor), c-KIT (receptor tyrosine kinase), and BCR-ABL [1]. Dasatinib is also a tyrosine kinase inhibitor; it can help block tyrosine kinase. It is a conventional anticancer drug for treating chronic myelogenous and acute lymphoblastic leukemia [2]. It is also beneficial for treating prostate cancer, nonHodgkin’s lymphoma, and metastatic breast cancer with many side effects. Despite a remarkable improvement in patient survival, some adverse effects of dasatinib, such as cardiovascular, hematologic, gastrointestinal, endocrine, and pulmonary toxicity, have been described. Some gastrointestinal side effects are nausea and vomiting, diarrhea, stomach discomfort, hemorrhagic colonic ulcers, acute hepatitis, anorexia, dyspepsia, and gastrointestinal bleeding resulting from platelet failure [3]. Due to its toxicity and substantial consumption of DAS, it is essential to accurately and quickly determine its concentration in the therapeutic window [4].
Up to now, there are a few techniques employed for the determination of DAS, including spectroscopy [5], chromatography [6], and voltammetry [7]. However, spectroscopic and chromatographic techniques have disadvantages, such as expensive equipment, sample pre-treatments, and time-consuming methods. In this framework, electroanalytical techniques are good alternatives with significant detection limits [8]. In this context, electrochemical techniques are excellent options, and voltammetric techniques are inexpensive, sensitive, and selective. Because of these benefits, voltammetric methods have often been employed to study redox reactions and analyze inorganic and organic compounds using a variety of electrodes. Nowadays, the creation of low cost, miniaturized electrochemical sensors for detecting various analytes is made possible by disposable screen-printed carbon electrodes (SPCEs) produced using thick-film technology [9, 10]. SPCEs as alternative to the standard electrochemical setups using conventional electrodes are available for electrochemical applications due to their low cost, easy and quick activating procedure, high chemical stability, disposability, portability, large potential window, short analysis time, simplicity, shorter sample pre-treatment processes, low background current with an economical substrate, and no need for time-consuming processes. One of the most notable benefits of SPCEs is the ability to analyze a trace amount of sample solution [11]. Hence, SPCEs are becoming a crucial tool in the creation of electrochemical sensors. Moreover, modifying electrochemical sensors with nanomaterials such as single-walled carbon nanotubes (SWCNTs) may improve their sensitivity and selectivity due to their large surface area (about 600 m2 g−1) and high electrical conductivity [12,13,14]. Compared to other carbon-based electrodes, their tiny diameter and long length enable them to be plugged into targets with superior electro-activity. SWCNTs have outstanding electrocatalytic activity in the redox behaviour of various chemicals because they transfer electrons more quickly than other carbon-based materials [15].
There are few studies on the voltammetric determination of dasatinib. Moghaddam et al. [16] and Kalambate et al. [17] reported that dasatinib was electrochemically detected at Fe3O4-SWCNTs 1-hexyl-3-methylimidazolium tetrafluoroborate-based paste electrode and Pd@Pt/MWCNT (mesoporous core–shell NPs supported on MWCNT) glassy carbon electrode, respectively. However, preparation of these electrodes (including synthesis of nanoparticles) took place approximately 20 h. Moreover, the reproducibility of the preparation is also the question since the nanostructured surface can be easily modified by external conditions. Thus, taking advantage of the merits of the SPCEs, we propose a fast determination method for DAS in pharmaceutical tablets using disposable SWCNT-modified screen-printed carbon electrodes based on the DAS oxidation signal. The technique is low-cost, sensitive, robust, and fast for screening of pharmaceutical samples at the point-of-care level.
Results and discussion
Characterization of SPCEs
SEM images of SPCEs were performed to investigate the surface morphology of the working electrode modified with nanoparticles. Figure 1A shows the surface of a bare screen-printed carbon electrode with a smooth morphology. But modification of the electrode surface with graphene oxide (GPHOX)/graphene nanoparticles (GPH) demonstrates stable and monolayer structures (Fig. 1B–C). Notably, carbon nanotube (CNT)/single-walled carbon nanotubes modified SPCEs exhibit a compact film with a tubular distribution structure of nanotubes on the surface of the screen-printed carbon electrodes (Fig. 1D–E). The surface of the SWCNT-SPCE was very uneven, which can be helpful in the enhancement of the electrode surface area. Subsequently, these results confirmed that the SPCE was modified with single-walled carbon nanotubes, which altered the electrode surface activity. Also, these results validate that the dispersion of SWCNT on the surface of the screen-printed carbon electrode was performed successfully. Also, the EDS of the SWCNT-modified working electrode is presented in Fig. 1F.
SWCNT-modified SPCE as the optimal SPCE for dasatinib quantitation
This work aims to select the best SPCE for studying DAS oxidation behaviour and fast determination of it in a pharmaceutical sample. The electrochemical behaviour of DAS has been analysed by measuring the cyclic voltammetry (CV) at different modified SPCEs at various pHs. In cyclic voltammetry, 100 µM DAS showed an oxidation peak at 0.6–0.8 V depending on the pH and type of SPCE when the potential range was between –1.5 and 1.5 V, with a scan rate of 100 mV s−1 in a 40 mM Britton–Robinson (BR) buffer. The absence of a reduction peak for DAS on the reverse scan, as shown in Fig. 2, indicated that the oxidation process is irreversible. These peaks refer to the anodic oxidation of dasatinib using bare and modified SPCEs.
Various types of SPCEs, including those based on bare carbon, modified SWCNTs, CNTs, GPH, or GPHOX, were evaluated as different working electrodes. The signal intensity obtained using various electrodes in accordance with the applicable approach is shown in Fig. 2A and Fig. S2. The SWCNT-modified SPCE was shown to give a greater peak intensity with a sharper peak. In contrast, the others exhibited broadened peaks, especially GPH and GPHOX-modified SPCEs. Hence, this kind of electrode proved to be the best. The difference in the electric charges of SWCNT-COOH-SPCE (negative charge due to the loss of the carboxylic group's proton) and the positive charge of DAS at this pH (pH 5.0) could explain the sensitivity of this compound's behavior over the electrode. The anodic peak current rose at the SWCNT-modified SPCE compared to the bare SPCE in Fig. 2B, showing the critical function of single-walled carbon nanotubes. This can be related to the increment of the active surface area of the electrode due to modification with SWCNTs which has a large surface area of about 600 m2 g−1, high electrical conductivity, and the accumulation of DAS on the surface of the modified SPCE. It shows the crucial role of SWCNTs in the oxidation process and electrode activity. SWCNTs can accelerate the electron transfer rate with excellent electrocatalytic behavior toward the oxidation of dasatinib.
To comprehend the interaction and oxidation behavior of DAS over the SPCE, it is crucial to understand how the positive charge of the DAS molecule changes with pH. Dasatinib API has two basic ionization constants (pKA), which are 6.8 and 3.1, and one weakly acidic pKA, which is 10.8, in a saturated solution in water with a pH of approximately 6.0 [18]. In this way, this study evaluated the effect of the pH values over a broad range from 3.0 to 10.0 of the BR buffer on the 100 µM dasatinib electrochemical behavior through cyclic voltammetry. It was observed that for DAS compound, peak 1a broadened as the supporting electrolyte's pH rose, and the voltammogram at pH 9.0 and pH 10.0 revealed two successive charge transfer processes, peak 1a at Ep1a = + 0.61 V and peak 2a at Ep2a = + 0.94 V for pH 9.0 and peak 1a at Ep1a = + 0.46 V and peak 2a at Ep2a = + 0.81 V for pH 10.0 (Fig. S3A). The buffer pH considerably influenced the anodic peak currents (Ipa) at the surface of SWCNT-COOH-SPCE. It was observed that with increasing pH, the peak current of the modified SWCNT electrode at pH 5.0 is higher than that of other types of SPCE and pH (Fig. 2B), probably related to the large effective surface area of SWCNTs. The Ipa of dasatinib steadily increased with the increment of pH from 3.0 to 5.0. They decreased afterward because of the peak broadening. The anodic peak current of dasatinib reaches a maximum at pH 5.0 (Fig. S3C). Also, with a rise in pH, the anodic peak potential of dasatinib slightly shifted toward the negative. According to these findings, protons have participated in their electrode reaction processes. Ep versus pH was plotted with a slope of approximately – 0.0602 V (Fig. S3B). Therefore, it was concluded that both peaks signify irreversible processes involving two protons and two electrons.
This finding is consistent with the known electrochemical reactions of dasatinib, as demonstrated by another research. It is well known that two electron and two proton processes contribute to the oxidation of dasatinib. It is suggested that dasatinib's thiazole moiety is involved in its oxidation. This may be referred to the creation of sulfoxide due to the transfer of two electrons and two protons from the sulfur atom of the thiazole ring in the presence of Britton–Robinson buffer (Fig. 2C) [7].
Then, the carryover effect was evaluated on bare and SWCNT-modified electrodes. The Britton–Robinson buffer and dasatinib signals were assessed on the same electrode. The RSD of the peak current was 14% for the bare carbon electrode and 0.2% for the SWCNT-modified electrode. Therefore, SWCNT-COOH-SPCEs were used in further studies.
Effect of scan rate at SWCNT-modified SPCE
The effect of the scan rate on DAS electro-oxidation was evaluated in the range of 20–200 mV s−1 on the surface of the SWCNT-modified electrode using the CV technique (Fig. 3A). The dependence of Neperian logarithm of peak current on the Neperian logarithm of sweep rate (ln Ip vs. ln v) is linear in which slope is 0.7499 ± 0.02 (Fig. 3B). It is indicated that the kinetics of the electrode process was controlled by adsorption, so the anodic oxidation of DAS on the surface of the SWCNT-COOH-SPCE is an adsorption-controlled electrochemical process. It is established that a slope is less than 0.5 is related to diffusion-controlled electrode processes, but if the slope is close to 1.0, the electrode process is adsorption-controlled [19,20,21,22,23,24]. It is described by the following equation [25]:
The effect of scan rate A CV of 100 µM dasatinib at SWCNT-modified SPCE; Britton–Robinson buffer pH 5.0; scan rates 20, 25, 50, 75, 100, 150, 200 mV s−1. B The plot of ln Ipa (Napierian logarithm of oxidation peak currents) vs. ln v (Napierian logarithm of scan rates). C Dependency of oxidation Epa (peak potentials) vs. ln v
According to Fig. 3C, the peak currents were found to increase with increasing the scan rate, with a shift in the oxidation peak potential of DAS towards a more positive window (anodic area) which confirms the irreversible nature of electrode processes. Figure 3C shows the dependence of Epa on the Neperian logarithm of the scan rate (Epa vs. ln v). If the electrochemical reaction is irreversible, then the Epa is independent of the scan rate. Therefore, it can be deduced that heterogeneous electron transfer in DAS electro-oxidation is irreversible because Epa increases with increasing scan rate. By increment of scan rate, the anodic peak potential Epa shifted towards more positive values, and a linear relationship was witnessed in the range of 20–200 mV s−1, as shown in Fig. 3C. The equation of this behaviour can be expressed as:
Additionally, for an adsorption-controlled and irreversible electrode process, the following Laviron equation between Epa vs. ln v can be used to determine the value of the reaction's total electron transfer coefficient [26]:
where α is the electron-transfer coefficient, n is the number of electrons, and v is the potential sweep rate. The other symbols have their usual meaning. Taking F = 96,485 C mol−1, T = 298 K, and R = 8.314 J K−1 mol−1, in this study, employing the dependence of anodic peak potential on the logarithm of the potential sweep rate (the slope = 0.0449), the value of total electron transfer coefficient (αn) was calculated to be 0.28 for DAS electro-oxidation.
α can be calculated based on the equation Ep/2 − Ep = 1.875 (RT/αF) [26, 27], where Ep and Ep/2 are the peak potential and the potential at which the current (Ip) is equal to half its peak value (Ip/2) in cyclic voltammogram, respectively. The value of α equals 0.13. Furthermore, the shape factor in the irreversible system is given by |Ep − Ep/2|= 47.7/αn (mV) [28,29,30], from which the number of electrons transferred in the electrochemical reaction of DAS (in the presence of Britton–Robinson buffer) were found to be approximately 2 (n = 2.1).
Optimized experimental SWCNT-modified SPCE
The calibration curve in Fig. 4A–B shows that the oxidation signal is linearly associated with dasatinib in the concentrations range of 0.1–100 µM, with a correlation coefficient of 0.9975. The limit of detection (LOD), calculated as signal/noise = 3.3, was estimated to be 0.06 µM. The limit of quantitation (LOQ), calculated as signal/noise = 10, was 0.19 µM. Based on the obtained results, the developed electrochemical sensor had relatively high sensitivity and good linearity. The linear range for determining DAS tablet solutions (as actual samples) was investigated from 0.1 to 100 µM with a correlation coefficient of 0.999 (Fig. 4C). The LOD, calculated as signal/noise = 3.3, and LOQ, calculated as signal/noise = 10, were estimated to be 0.07 µM and 0.22 µM, respectively (Table 1). The amount of dasatinib found was 70 mg/tablet, representing a relative error of 6%.
A Concentration effect of DAS detection by SWV method in concentration range (100, 70, 30, 10, 7, 3, 0.7, 0.3, and 0.1 µM) in Britton–Robinson buffer pH 5.0 by SWCNT-modified SPCEs. Step potential 0.005 V, scan rate 100 mV s−1. B Calibration curve related to SWV analysis of DAS. C Calibration curve related to SWV analysis of real sample dasatinib tablet solutions
Therefore, the carbon-based screen-printed electrodes, a new generation of electrodes, were used to determine the anticancer drug dasatinib. According to the obtained results, modification of SPCEs with nanomaterials resulted in a better response for dasatinib than the bare ones. And among the different types of modified SPCEs, single-walled carbon nanotube-modified electrodes gave the highest peak currents for dasatinib detection. SWCNTs improve the electrochemically active area and enhance the electronic transfer attributes. The tubular structure of SWCNTs on the SPCE surface was very uneven, which led to the enhancement of the surface area of the electrode. The developed platform showed a good electrochemical response towards dasatinib with LODs of 0.06 µM. The established electrochemical sensor is among the superior reported electrochemical sensors (Table 2) in terms of repeatability, low detection limit, and broad linear range. It should be highlighted that the excellent performance of the SWCNT was related to its outstanding properties like high electrical conductivity and large surface area, which pave the way for fast electrons. The results showed that the developed electrochemical sensor also had relatively high sensitivity and good linearity in detecting dasatinib in actual samples, too. Consequently, the single-walled carbon nanotube-modified screen-printed electrodes could be used for the sensitive and quick analysis of electrochemically active pharmaceutical substances.
Reproducibility, selectivity, and stability of the sensor
Six sensors were chosen randomly to detect dasatinib under the same experimental conditions. The proposed method’s repeatability (relative standard deviation) was assessed by measuring square-wave voltammetry (SWV) of 100 µM DAS in Britton–Robinson buffer pH 5.0 (intraday stability) through a SWCNT-modified electrode. The relative standard deviation (RSD%) was 1.89% to determine DAS in one day.
The interference study serves a significant role in analytical chemistry. To this end, the sensor's selectivity for determining DAS was investigated through the influences of some coexisting interferences like different organic and inorganic compounds such as lactose, sucrose, glucose, SO42−, and CO32− in the real samples. Figure 5 displays the SWV measurements of DAS and its mixtures with various interfering species. As can be seen, these compounds had almost no effect on the peak current of DAS, with a difference of less than 1.8% in peak current (%ΔI) between the peak current of DAS with and without interference. It demonstrated that the suggested sensor is highly selective for dasatinib detection. It should be noted that using glucose as an interfering agent broadened the DAS peak and showed two consecutive charge transfer reactions (Fig. 5A).
Conclusions
Herein, sensitive individual electrochemical determination of dasatinib is introduced on a SWCNT-modified SPCE platform. Compared to other electrochemical sensors with various nanoparticles modification such as GPH, GPHOX, CNT, and bare electrodes, the SWCNT-modified SPCE showed an excellent function for dasatinib detection.
Dasatinib could demonstrate an irreversible signal with good sensitivity at a surface of SWCNT-modified SPCE compared to others. SWCNTs-modified SPCE showed good catalytic activity of the oxidation signal of the DAS anticancer drug and improved its signal. This work has exhibited that dasatinib has only one oxidation peak at about + 0.8 V at SWCNT-modified SPCE in Britton Robinson buffer solution, pH 5.0, and the electrochemical oxidation happens through step by two electrons and protons. The quantitative measurement of dasatinib with the SWV technique was optimized to voltammetric parameters. The developed platform showed a great electrochemical response towards DAS with an excellent detection limit and selectivity. Also, based on the results, there was a good linear relationship between the concentration range of DAS and its oxidation peak current. The results showed that our sensor could detect low dasatinib concentrations in tablet solutions (as actual samples).
Experimental
Boric acid (99.50%, ACS reagent), phosphoric acid (≥ 85%, ACS reagent), acetic acid (≥ 99.8%, ACS reagent), and methanol (≥ 99.8%, ACS reagent) were supplied from Sigma–Aldrich (St. Louis, MO). The dasatinib drug was purchased from Sigma–Aldrich (Prague, Czech Republic). All solutions were prepared using ultrapure water (resistivity of 18.2 MΩ cm, MilliQ, Millipore, France). The Olomouc University Hospital kindly provided samples of the dasatinib tablet. Commercial buffer solutions with pH levels of 4.0, 7.0, and 10.0 were used to calibrate the pH electrode. All experiments were done at room temperature.
Britton–Robinson buffer was prepared by an equal volume of 40 mM H3BO3:H3PO4:CH3COOH. The pH was adjusted to 3–10 with 0.1 mM NaOH. The 100 µM dasatinib stock solution was prepared by dissolving it into ethanol/water (1:1) solution and ultrasonication for 30 min. Britton–Robinson buffer was used as the supporting electrolyte. The drug solutions were kept at 4 °C. For dasatinib detection, 60 µL of dasatinib solution was cast on the surface of SPCE and analyzed using electrochemical methods.
Preparation of standards and samples calibration
Britton–Robinson buffer solution pH 5.0 was selected as the optimum buffer pH. Hence, dasatinib concentrations varying from 0.1 µM to 100 µM were prepared in Britton–Robinson buffer solution pH 5.0. Then, for the actual sample, 9.7 mg of the powdered dasatinib tablet was dissolved in 2 mL 50% aqueous ethanol (35 °C) and sonicated for 15 min. After filtration, 30 µL of the solution was diluted in Britton–Robinson buffer solution (pH = 5.0) and used to analyze actual samples using the standard addition method of 40 µL of the dasatinib tablet solution was mixed with 20 µL of various concentrations of standard solutions.
For dasatinib quantitation, all electrochemical measurements (CV and SWV) were made on the surface of SPCEs. SWV was chosen as a more sensitive electrochemical method with a much lower background current, a higher current sensitivity, and a better resolution than CV. Under optimal conditions, signals were recorded for square-wave voltammetric investigation in the potential range of 0.2 to (+ 1.5) V with 10.0 Hz using SWCNT-modified SPCEs. All experiments were repeated three times.
Instruments
Electrochemical measurements were performed on the Metrohm Potentiostat PGSTAT101 AUTOLAB (Prague, Czech Republic) with NOVA 2.1 software. Six different screen-printed electrodes were purchased from Metrohm Dropsens (Prague, Czech Republic). The screen-printed electrochemical cell consists of a carbon working electrode (4 mm diameter), a silver or Ag/AgCl reference electrode, and an auxiliary carbon electrode. SPCE studies included electrodes for bare, single-walled carbon nanotubes (SWCNT)-modified, graphene (GPH)-modified, graphene oxide (GPHOX)-modified, and carbon nanotubes (CNT)-modified SPCEs. For DPV measurements, the step potential of 0.005 V and the scan rate of 100 mV s−1 were applied. The 10.0 Hz frequency was also used for SWV measurements. The surface morphology of the screen-printed carbon electrodes was characterized by Hitachi SU6600 SEM (Hitachi, Japan) scanning electron microscope (SEM). EDS (Energy dispersive spectroscopy) coupled with the SEM equipment was also employed to analyze the chemical compositions of the SPCEs.
Data availability statement
The data are available from the authors upon reasonable request.
References
Lindauer M, Hochhaus A (2018) Dasatinib. Small molecules in hematology. Springer, Cham, p 29
Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E (2006) New Engl J Med 354:2531
Nekoukar Z, Moghimi M, Salehifar E (2021) Blood Res 56:229
Chen HX, Cleck JN (2009) Nat Rev Clin Oncol 6:465
Sankar DG, Rajeswari A, Babu AN, Krishna MV (2009) Asian J Chem 21:5777
Roche S, McMahon G, Clynes M, O’Connor R (2009) J Chromatogr B 877:3982
Jesus CS, Diculescu VC (2015) J Electroanal Chem 752:47
Aydoğdu Tığ G, Günendi G, Pekyardımcı Ş (2017) J Appl Electrochem 47:607
Santiago LM, Bejarano-Nosas D, Lozano-Sanchez P, Katakis I (2010) Analyst 135:1276
Mata D, Bejarano D, Botero M, Lozano P, Constantí M, Katakis I (2010) Electrochim Acta 55:4261
Koç Y, Morali U, Erol S, Avci H (2021) Turk J Chem 45:1895
Tiwari JN, Vij V, Kemp KC, Kim KS (2016) ACS Nano 10:46
Hassanpour S, Mokhtarzadeh A, Hasanzadeh M, Hejazi M, Baradaran B (2019) Nanomaterials for use in apta-assays. Handbook of smart materials in analytical chemistry. Wiley, Chichester, UK, p 243
Birch ME, Ruda-Eberenz TA, Chai M, Andrews R, Hatfield RL (2013) Ann Occup Hyg 57:1148
Saleh Ahammad AJ, Lee JJ, Rahman MA (2009) Sensors (Basel) 9:2289
Moghaddam A, Zamani HA, Karimi-Maleh H (2021) Micromachines (Basel) 12:437
Kalambate PK, Li Y, Shen Y, Huang Y (2019) Anal Methods 11:443
Lakka NS, Kuppan C, Srinivas KS, Yarra R (2020) Chromatographia 83:947
Kissinger P, Heineman WR (2018) Laboratory techniques in electroanalytical chemistry, revised and expanded. CRC Press
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, vol 2. Wiley, UK, p 580
Hasanzadeh M, Mokhtari F, Jouyban-Gharamaleki V, Mokhtarzadeh A, Shadjou N (2018) J Mol Recognit 31:e2717
Hassanpour S, Saadati A, Hasanzadeh M (2020) J Mol Recognit 33:e2817
Feyziazar M, Hasanzadeh M, Farshchi F, Saadati A, Hassanpour S (2020) React Funct Polym 146:104402
Nahr AS, Hassanpour S, Hasanzadeh M (2020) Anal Methods 12:1639
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley, New York
Laviron E (1979) J Electroanal Chem Interf Electrochem 101:19
García MG, García AC (1995) Bioelectrochem Bioenerg 38:389
Lee B-G, Rhyu K-B, Yoon K-J (2009) Bull Korean Chem Soc 30:2457
Brett CMA, Brett AMO (1993) Electrochemistry: principles, methods, and applications. Oxford University Press
Faulkner LR, Bard AJ (2002) Electrochemical methods: fundamentals and applications. Wiley, New York
Alavi-Tabari SA, Khalilzadeh MA, Karimi-Maleh H (2018) J Electroanal Chem 811:84
Tahernejad-Javazmi F, Shabani-Nooshabadi M, Karimi-Maleh H (2018) New J Chem 42:16378
Bayraktepe DE, Polat K, Yazan Z (2018) J Turk Chem Soc, Sect A: Chem 5:381
Karimi-Maleh H, Shojaei AF, Tabatabaeian K, Karimi F, Shakeri S, Moradi R (2016) Biosens Bioelectron 86:879
Acknowledgements
This work was supported by the Palacký University Olomouc (project IGA_PrF_2022_023). Also, we gratefully acknowledge Klára Čépe and Jana Stráská from CATRIN–RCPTM (Palacký University Olomouc) for the SEM measurements.
Funding
Open access publishing supported by the National Technical Library in Prague. Univerzita Palackého v Olomouci, IGA_PrF_2022_023, Jan Petr.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassanpour, S., Petr, J. A disposable electrochemical sensor based on single-walled carbon nanotubes for the determination of anticancer drug dasatinib. Monatsh Chem 154, 1061–1069 (2023). https://doi.org/10.1007/s00706-023-03043-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-023-03043-w