Abstract
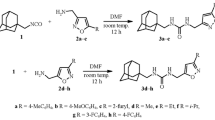
Potentially biologically active urea derivatives have been prepared in an efficient one-pot procedure from 1,2,3,4-tetrahydronaphthalene-2-carboxylic acids and characterized via several spectroscopy techniques. Ideal reaction times and temperatures, as well as the absence of metal catalysts and time-consuming and expensive purification chromatographic conditions, allowed the products to be in good yield. The obtained unsymmetrical urea derivatives in an economical and efficient synthetic methodology would be of great interest to be used for potential applications related to drug design, pharmaceutical, and medicinal chemistry.
Graphical abstract




Similar content being viewed by others
References
Paget CJ, Kisner K, Stone RL, DeLong DC (1969) J Med Chem 12:1016
Schroeder MC, Hamby JM, Connolly CJC, Grohar PJ, Winters RT, Barvian MR, Moore CW, Boushelle SL, Crean SM, Kraker AJ (2001) J Med Chem 44:1915
Li H-Q, Lv P-C, Yan T, Zhu H-L (2009) Anti-Cancer Agent Med Chem 9:471
Wróbel TM, Kiełbus M, Kaczor AA, Krystof V, Karczmarzyk Z, Wysocki W, Fruzinski A, Krol SK, Grabarska A, Stepulak A, Matosiuk D (2016) J Enzym Inhib Med Chem 31:608
Francisco GD, Li Z, Albright JD, Eudy NH, Katz AH, Petersen PJ, Labthavikul P, Singh G, Yang Y, Rasmussen BA (2004) Bioorg Med Chem Lett 14:235
Pandurangan K, Kitchen JA, Blasco S, Paradisi F, Gunnlaugsson T (2014) Chem Commun 50:10819
Zhang Y, Anderson M, Weisman JL, Lu M, Choy CJ, Boyd VA, Price J, Sigal M, Clark J, Connelly M (2010) ACS Med Chem Lett 1:460
North EJ, Scherman MS, Bruhn DF, Scarborough JS, Maddox MM, Jones V, Grzegorzewicz A, Yang L, Hess T, Morisseau C (2013) Bioorg Med Chem 21:2587
Njoroge FG, Chen KX, Shih NY, Piwinski JJ (2008) Acc Chem Res 41:50
Zeldin RK, Petruschke RA (2004) J Antimicrob Chemother 53:4
Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M (2008) Clin Cancer Res 14:5459
Gould TD, Einat H, Bhat R, Manji HK (2004) Int J Neuropsychopharmacol 7:387
Berg S, Hellberg S (2003) Preparation of N-(4-methoxybenzyl)-N’-(5-nitro-1,3-thiazol-2-yl)urea for treating conditions associated with glycogen-synthase kinase-3 (GSK3) Patent WO. 2003004478 Chem Abstr 138:106690
Abe H, Matsunaga S, Takekawa S, Watanabe M (2004) Preparation of indole amino acid derivatives as somatostatin agonists or antagonists Patent WO 2004046107. Chem Abstr 141:23903
Lintner K (2004) Method for preparing cosmetic or dermopharmaceutical compositions comprising tyramine derivatives and use thereof Patent WO 2004002941. Chem Abstr 140:77297
Özgeris B, Göksu S, Polat Köse L, Gülçin I, Salmas RE, Durdagi S, Tümer F, Supuran CT (2016) Acetylcholinesterase and carbonic anhydrase inhibitory properties of novel urea and sulfamide derivatives incorporating dopaminergic 2-aminotetralin scaffolds. Bioorg Med Chem 24(10):2318
Kadyrov R, Riermeier TH (2003) Angew Chem Int Ed 42:5472
Molinoff PB, Axelrod J (1971) Annu Rev Biochem 40:465
Zhou Q-Y, Palmiter RD (1995) Cell 83:1197
Carlsson A (1972) Acta Neurol Scand Suppl 51:11
Broadley KJ (2010) Pharmacol Ther 125:363
Giladi N, Boroojerdi B, Korczyn AD, Burn DJ, Clarke CE (2007) Mov Disord 22:2398
Sixel-Döring F, Trenkwalder C (2010) Expert Opin Pharmacother 11:649
Tang G, Wong JC, Zhang W, Wang Z, Zhang N, Peng Z, Zhang Z, Rong Y, Li S, Zhang M (2014) J Med Chem 57:8026
Duspara PA, Islam MS, Lough AJ, Batey RA (2012) J Org Chem 77:10362
Tundo P, Selva M (2002) Acc Chem Res 35:706
Grzyb JA, Shen M, Yoshina-Ishii C, Chi W, Brown RS, Batey RA (2005) Tetrahedron 61:7153
McMorris TC, Chimmani R, Alisala K, Staake MD, Banda G, Kelner MJ (2010) J Med Chem 53:1109
Ready JM, Nijhawan D, Gonzales SS, Theodoropoulos P (2015) Preparation of benzothiophenes and other compounds and methods and compositions for selective and targeted cancer therapy Patent WO 2015035051. Chem Abstr 162:413384
Diaz DJ, Darko AK, McElwee-White L (2007) Eur J Org Chem 2007:4453
Deng Y, Yan Z, Liu J, Liu Y, Sun L (2017) Arch Med Res 48:333
Krishnakumar V, Chatterjee B, Gunanathan C (2017) Inorg Chem 56:7278
Pitushkin DA, Burmistrov VV, Butov GM (2020) Russ J Org Chem 56:1336
Liang J, Cochran JE, Dorsch WA, Davies I, Clark MP (2016) Org Process Res Dev 20:965
Göksu S, Kazaz C, Sütbeyaz Y, Seçen H (2003) Helv Chim Acta 86:3310
Göksu S, Seçen H, Sütbeyaz Y (2006) Helv Chim Acta 89:270
Akbaba Y, Türker Balaydın H, Göksu S, Şahin E, Menzek A (2010) Helv Chim Acta 93:1127
Balaydin HT, Akbaba Y, Menzek A, Sahin E, Göksu S (2009) ARKIVOC 14:75
Öztaşkın N, Göksu S, Secen H (2011) Synth Commun 41:2017
Mal D, Dey S (2006) Tetrahedron 62:9589
Anagi TY, Ikuchi KK, Akeuchi HT, Shikawa TI, Ishimura TN (2001) Chem Pharm Bull 49:340
Acknowledgements
I would like to thank Atatürk University for taking NMR spectra and also to thank Erzurum Technical University High Technology Research Center for research conditions. I would also like to thank Prof. Dr. Süleyman Göksu for his helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akbaba, Y. A straightforward synthesis of potentially biologically active novel urea derivatives from 1,2,3,4-tetrahydronaphthalene-2-carboxylic acids. Monatsh Chem 153, 1251–1259 (2022). https://doi.org/10.1007/s00706-022-02982-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-022-02982-0