Abstract
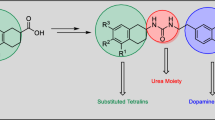
The review presents and summarizes comprehensive data starting from 2000 on the synthesis of ureas containing alkylaromatic moieties. The advantages and drawbacks of various synthetic methods were highlighted. The biological activity was covered for some representatives of this class of compounds.
Similar content being viewed by others
References
E. W. Schmidt, M. K. Harper, D. J. Faulkner, J. Nat. Prod., 1997, 60, 779.
T. Sano, K. Kaya, Tetrahedron Lett., 1995, 36, 5933.
R. H. Chen, A. M. Buko, D. N. Whitern, J. B. Mcalpine, J. Antibiot. (Tokyo), 1989, 42, 512.
T. Temal, H. Jary, M. Auberval, S. Lively, D. Guédin, J. Vevert, P. Deprez, Bioorg. Med. Chem. Lett., 2013, 23, 2455.
P. Deprez, T. Temal, H. Jary, M. Auberval, S. Lively, D. Guédin, Bioorg. Med. Chem. Lett., 2013, 23, 2455.
P. M. Wehn, P. E. Harrington, T. J. Carlson, J. Davis, P. Deprez, C. H. Fotsch, M. P. Grillo, J. Y.-L. Lu, S. Morony, K. Pattabiraman, S. F. Poon, J. D. Reagan, D. J. St. Jean, Jr., T. Temal, M. Wang, Y. Yang, C. Henley, S. E. Lively, Bioorg. Med. Chem. Lett., 2013, 23, 6625.
U. R. Mane, D. Mohanakrishnan, D. Sahal, P. R. Murumkar, R. Giridhar, M. Ram, Eur. J. Med. Chem., 2014, 79, 422.
V. G. DeVries, J. D. Bloom, M. D. Dutia, A. S. Katocs, E. E. Largis, J. Med. Chem., 1989, 32, 2318.
S. L. Nowotarski, B. Pachaiyappan, S. L. Holshouser, C. J. Kutz, Y. Li, Y. Huang, S. K. Sharma, R. A. Casero, P. M. Woster, Bioorg. Med. Chem., 2015, 23, 1601.
A. Esteves-Souza, K. Pissinate, M. D. Graça Nascimento, N. F. Grynberg, A. Echevarria, Bioorg. Med. Chem., 2006, 14, 492.
J. A. Kowalski, A. D. Swinamer, I. Muegge, A. B. Eldrup, A. Kukulka, C. L. Cywin, S. De Lombaert, Bioorg. Med. Chem. Lett., 2010, 20, 3703.
M. Manickam, T. Pillaiyar, P. Boggu, E. Venkateswararao, H. B. Jalani, N.-D. Kim, S. K. Lee, J. S. Jeon, S. K. Kim, S.-H. Jung, Eur. J. Med. Chem., 2016, 117, 113.
M. L. Sanders, I. O. Donkor, Bioorg. Med. Chem. Lett., 2006, 16, 1965.
I. Gallou, Org. Prep. Proced. Int., 2007, 39, 355.
T. P. Vishnyakova, I. A. Golubeva, E. V. Glebova, Russ. Chem. Rev., 1985, 54, 249.
D. F. Kutepov, Russ. Chem. Rev., 1962, 31, 633.
F. Bigi, R. Maggi, G. Sartori, Green Chem., 2000, 2, 140.
N. F. Lazareva, B. A. Gostevskii, Russ. Chem. Bull., 2017, 66, 2199.
A. Nefzi, N. A. Ong, R. A. Houghten. Tetrahedron Lett., 2000, 41, 5441.
M. Manickam, H. B. Jalani, T. Pillaiyar, N. Sharma, P. R. Boggu, E. Venkateswararao, Y.-J. Lee, E.-S. Jeon, S.-H. Jung, Eur. J. Med. Chem., 2017, 134, 379.
T. Mohy El Dine, S. Chapron, M.-C. Duclos, N. Duguet, F. Popowycz, M. Lemaire, Eur. J. Org. Chem., 2013, 2013, 5445.
H.-M. Wang, H.-X. Li, X.-Y. Yu, Z.-G. Ren, J.-P. Lang, Tetrahedron, 2011, 67, 1530.
J. C. Anderson, R. Bou-Moreno, Tetrahedron, 2010, 66, 9182.
J. C. Anderson, R. B. Moreno, Org. Biomol. Chem., 2012, 10, 1334.
S. R. Jagtap, Y. P. Patil, A. G. Panda, B. M. Bhanage, Synth. Commun., 2009, 39, 2093.
F. Saliu, B. Rindone, Tetrahedron Lett., 2010, 51, 6301.
S. Fujita, B. M. Bhanage, M. Arai, Chem. Lett., 2004, 33, 742.
S. Fujita, B. M. Bhanage, H. Kanamaru, M. Arai, J. Mol. Catal. A Chem., 2005, 230, 43.
R. Ballini, D. Fiorini, R. Maggi, P. Righi, G. Sartori, R. Sartorio, Green Chem., 2003, 5, 396.
P. A. Duspara, M. S. Islam, A. J. Lough, R. A. Batey, J. Org. Chem., 2012, 77, 10362.
M. Wang, J. Han, X. Si, Y. Hu, J. Zhu, X. Sun, Tetrahedron Lett., 2018, 59, 1614.
K. Smith, G. El-Hiti, M. Alshammari, Synthesis, 2012, 44, 2013.
K. Smith, G. A. El-Hiti, M. B. Alshammari, J. Org. Chem., 2012, 77, 11210.
S. H. Kim, S. H. Hong, Org. Lett., 2016, 18, 212.
D. J. Díaz, K.-G. Hylton, L. McElwee-White, J. Org. Chem., 2006, 71, 734.
L. Jin, D. S. Weinberger, M. Melaimi, C. E. Moore, A. L. Rheingold, G. Bertrand, Angew. Chem., Int. Ed., 2014, 53, 9059.
K. Orito, M. Miyazawa, T. Nakamura, A. Horibata, H. Ushito, H. Nagasaki, M. Yuguchi, S. Yamashita, T. Yamazaki, M. Tokuda, J. Org. Chem., 2006, 71, 5951.
J. H. Park, J. C. Yoon, Y. K. Chung, Adv. Synth. Catal., 2009, 351, 1233.
S. L. Peterson, S. M. Stucka, C. J. Dinsmore, Org. Lett., 2010, 12, 1340.
D. Chaturvedi, N. Mishra, V. Mishra, Monatsh. Chem. — Chem. Mon., 2008, 139, 267.
C. Wu, H. Cheng, R. Liu, Q. Wang, Y. Hao, Y. Yu, F. Zhao, Green Chem., 2010, 12, 1811.
D.-L. Kong, L.-N. He, J.-Q. Wang, Synlett, 2010, 2010, 1276.
A. V. Bogdanov, L. I. Musin, V. F. Mironov, Arkivoc, 2015, 2015, 362.
D. Yamauchi, T. Nishimura, H. Yorimitsu, Angew. Chem., Int. Ed., 2017, 56, 7200.
F. Li, C. Sun, H. Shan, X. Zou, J. Xie, ChemCatChem, 2013, 5, 1543.
S. C. Ghosh, C. C. Li, H. C. Zeng, J. S. Y. Ngiam, A. M. Seayad, A. Chen, Adv. Synth. Catal., 2014, 356, 475.
Z. Li, Z. Wang, W. Zhu, Y. Xing, Y. Zhao, Synth. Commun., 2005, 35, 2325.
E. A. Chugunova, N. I. Akylbekov, A. D. Voloshina, N. V. Kulik, V. V. Zobov, V. M. Babaev, N. V. Gavrilov, A. R. Burilov, Synth. Commun., 2016, 46, 1560.
A. V. Smolobochkin, A. S. Gazizov, A. R. Burilov, M. A. Pudovik, Russ. J. Org. Chem., 2015, 85, 1779.
A. V. Smolobochkin, A. S. Gazizov, Yu. K. Voronina, A. R. Burilov, M. A. Pudovik, Russ. Chem. Bull., 2016, 65, 731.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on the materials of the V All-Russian Organic Chemistry Conference (ROCC-V) (September 10–14, 2018, Vladikavkaz, Russia).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 0662–0670, April, 2019.
Rights and permissions
About this article
Cite this article
Smolobochkin, A.V., Gazizov, A.S., Burilov, A.R. et al. Ureas bearing alkylaromatic moieties: their synthesis and biological activity. Russ Chem Bull 68, 662–670 (2019). https://doi.org/10.1007/s11172-019-2473-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-019-2473-8