Abstract
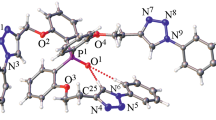
New hybrid tripodal propeller ligands on the triphenylphosphine oxide platform with triazole rings in the side arms and alkyl and aryl substituents in the triazole fragments have been synthesized by the click reaction. Composition and structure of the prepared compounds have been established by vibrational (IR, Raman) and multinuclear (1H, 13C, 31P) NMR spectroscopy, elemental analysis, and mass spectrometry. Coordination and extraction properties of the prepared compounds toward Pd(II) have been studied by the example of one of the ligands.
Graphic abstract








Similar content being viewed by others
References
Sahoo SK, Kim G-D, Choi H-J (2016) J Photochem Photobiol C Photochem Rev 30
Silva F, Fernandes C, Campello MPC, Paulo A (2017) Polyhedron 125:186
Lim J, Lynch VM, Edupuganti R, Ellington A, Anslyn EV (2016) Dalton Trans 45:10585
Phanopoulos A, Miller PW, Long NJ (2015) Coord Chem Rev 299:39
Batke S, Sietzen M, Wadepohl H, Ballmann J (2014) Inorg Chem 53:4144
Saad FA, Buurma NJ, Amoroso AJ, Knight JC, Kariuki BM (2012) Dalton Trans 41:4608
Gale PA (2011) Acc Chem Res 44:216
Khansari ME, Johnson CR, Basaran I, Nafis A, Wang J, Leszczynski J, Hossain MA (2015) RSC Adv 5:17606
Beletskiy EV, Wang XB, Kass SR (2016) J Phys Chem A 120:8309
Gale PA, Davis JT, Quesada R (2017) Chem Soc Rev 46:2497
Mazik M (2009) Chem Soc Rev 38:935
Stewart CD, Pedraza M, Arman H, Fan H-J, Schilling EL, Szpoganicz B, Musie GT (2015) J Inorg Biochem 149:25
Stewart CD, Arman H, Bawazir H, Musie GT (2014) Inorg Chem 53:10974
Leoncini A, Ansari SA, Mohapatra PK, Boda A, Ali SM, Sengupta A, Huskens J, Verboom W (2017) Dalton Trans 46:1431
Coburn KM, Hardy DA, Patterson MG, McGraw SN, Peruzzi MT, Boucher F, Beelen B, Sartain HT, Neils T, Lawrence CL, Staples RJ, Werner EJ, Biros SM (2016) Inorg Chim Acta 449:96
Sartain HT, McGraw SN, Lawrence CL, Werner EJ, Biros SM (2015) Inorg Chim Acta 426:126
Matloka K, Sah AK, Peters MW, Srinivasan P, Gelis AV, Regalbuto M, Scott MJ (2007) Inorg Chem 46:10549
Sharova EV, Artyushin OI, Turanov AN, Karandashev VK, Meshkova SB, Topilova ZM, Odinets IL (2012) Centr Eur J Chem 10:146
Kudryavtsev IY, Baulina TV, Pasechnik MP, Aysin RR, Matveev SV, Petrovskii PV, Nifant’ev EE (2013) Russ Chem Bull 62:1086
Kudryavtsev IY, Baulina TV, Pasechnik MP, Matveev SV, Matveeva AG (2014) Phosphorus. Sulfur Silicon Relat Elem 189:946
Matveeva AG, Kudryavtsev IY, Pasechnik MP, Vologzhanina AV, Baulina TV, Vavina AV, Sukat GY, Matveev SV, Godovikov IA, Turanov AN, Karandashev VK, Brel VK (2018) Polyhedron 142:71
Turanov AN, Matveeva AG, Kudryavtsev IY, Pasechnik MP, Matveev SV, Godovikova MI, Baulina TV, Karandashev VK, Brel VK (2019) Polyhedron 161C:276
Dam HH, Reinhoudt DN, Verboom W (2007) Chem Soc Rev 36:367
Aromí G, Barrios LA, Roubeau O, Gamez P (2011) Coord Chem Rev 255:485
Huang D, Zhao P, Astru D (2014) Coord Chem Rev 272:145
Schulze B, Schubert US (2014) Chem Soc Rev 43:2522
Kacprzak K, Skiera I, Piasecka M, Paryzek Z (2016) Chem Rev 116:5689
Lukashev NV, Grabovyi GA, Erzunov DA, Kazantsev AV, Latyshev GV, Averin AD, Beletskaya IP (2017) Beilstein J Org Chem 13:564
Keivanloo A, Lashkari S, Sepehri S, Bakherad M, Abbaspour S (2020) Monatsh Chem 151:935
Schweinfurth D, Pattacini R, Strobel S, Sarkar B (2009) Dalton Trans 42:9291
Lo WKC, Huff GS, Cubanski JR, Kennedy ADW, McAdam CJ, McMorran DA, Gordon KC, Crowley JD (2015) Inorg Chem 54:1572
Hurtado J, Rojas RS, Pérez EG, Valderrama M (2013) J Chil Chem Soc 58:1534
Crowley JD, Gavey EL (2010) Dalton Trans 39:4035
Turanov AN, Karandashev VK, Artyushin OI, Sharova EV, Genkina GK, Yarkevich AN (2014) Solvent Extr Ion Exch 32:669
Nozoe A, Morisada S, Ohto K, Kawakita H (2014) Solvent Extr Ion Exch 33:56
Turanov AN, Karandashev VK, Sharova EV, Genkina GK, Artyushin OI (2015) RSC Adv 5:27640
Kudryavtsev IY, Bykhovskaya OV, Aladzheva IM, Baulina TV, Brel VK (2017) Russ J Gen Chem 87:2744
Livant PD, Mao J, Webb TR (1996) Acta Cryst C52:2924
Bykhovskaya OV, Matveeva AG, Pasechnik MP, Vologzhanina AV, Matveev SV, Kudryavtsev IY, Baulina TV, Brel VK (2019) Russ J Gen Chem 89:2400
Baulina TV, Kudryavtsev IY, Smolyakov AF, Pasechnik MP, Brel VK (2018) Heteroat Chem 29:e21454
Baulina TV, Pasechnik MP, Kudryavtsev IY, Bykhovskaya OV, Sukat GY, Smol’yakov AF, Anikina LV, Brel VK (2020) J Mol Struct 1217:128324
Pasechnik MP, Matveeva AG, Lyssenko KA, Aysin RR, Smol’yakov AF, Zubavichusc YV, Godovikov IA, Goryunov EI (2019) J Mol Struct 1175:874
Durrell AC, Gray HB, Hazari N, Incarvito CD, Liu J, Yan EC-Y (2010) Cryst Growth Des 10:1482
Nakamoto K (1997) IR and Raman Spectra of Inorganic and Coordination Compounds. Wiley, New York
Kozlov VA, Aleksanyan DV, Korobov MV, Avramenko NV, Aysin RR, Maloshitskaya OA, Korlyukov AS, Odinets IL (2011) Dalton Trans 40:8768
Armarego WLF (2017) Purification of Laboratory Chemicals. Elsevier, Amsterdam
Gel’man NE, Terent’eva EA, Shanina TM, Kiparenko LM, Rezl V (1987) Methods of Quantitative Organic Elemental Microanalysis. Khimiya, Moscow
Sheldrick GM (2015) Acta Cryst A71:3
Spek AL (2015) Acta Cryst C71:9
Sheldrick GM (2015) Acta Cryst C71:3
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339
Acknowledgements
The work was financially supported by the Russian Science Foundation (project no. 20–13–00329). Spectral studies were carried out using the equipment of the Center for Molecular Structure Studies, INEOS RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kudryavtsev, I.Y., Bykhovskaya, O.V., Matveeva, A.G. et al. New tripodal ligand on the triphenylphosphine oxide platform with 1,2,3-triazole side arms: synthesis, structure, coordination, and extraction properties. Monatsh Chem 151, 1705–1713 (2020). https://doi.org/10.1007/s00706-020-02702-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02702-6