Abstract
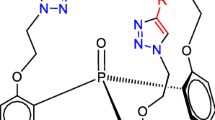
The Mitsunobu reaction of tris(2-hydroxyphenyl)phosphine oxide with 3-butyn-1-ol has afforded bis[2-(3′′-butynyloxy)phenyl](2′-hydroxyphenyl)phosphine oxide, which has been converted into the corresponding triazole via click reaction with PhN3. Asymmetric tripodal ligand containing three 1,2,3-triazole groups in the molecule has been prepared from this triazole via the reaction with propargyl bromide and PhN3. Structure of the ligand has been elucidated by means of single-crystal X-ray diffraction analysis.



Similar content being viewed by others
REFERENCES
Jevric, M., Zheng, T., Meher, N.K., Fettinger, J.C., and Mascal, M., Angew. Chem. Int. Ed., 2011, vol. 50, no. 3, p. 717. https://doi.org/10.1002/anie.201006470
Long, S.R., Lin, C.-Y., and Anslyn, E.V., J. Coord. Chem., 2017, vol. 70, no. 1, p. 1. https://doi.org/10.1080/00958972.2016.1262949
Lukashev, N.V., Grabovyi, G.A., Erzunov, D.A., Kazantsev, A.V., Latyshev, G.V., Averin, A.D., and Beletskaya, I.P., Beilstein J. Org. Chem., 2017, vol. 13, p. 564. https://doi.org/10.3762/bjoc.13.55
Neumajer, G., Tóth, G., Béni, S., and Noszál, B., Cent. Eur. J. Chem., 2014, vol. 12, no. 1, p. 115. https://doi.org/10.2478/s11532-013-0351-z
Grewal, S., Roy, S., Kumar, H., Saraswat, M., Bari, N.K., Sinha, S., and Venkataramani, S., Catal. Sci. Technol., 2020, vol. 10, no. 20, p. 7027. https://doi.org/10.1039/D0CY01090A
Jain, A., Jain, Y., Gupta, R., and Agarwal, M., J. Fluor. Chem., 2018, vol. 212, p. 153. https://doi.org/10.1016/j.jfluchem.2018.06.005
Erzunov, D.A., Latyshev, G.V., Averin, A.D., Beletskaya, I.P., and Lukashev, N.V., Eur. J. Org. Chem., 2015, vol. 2015, no. 28, p. 6289. https://doi.org/10.1002/ejoc.201500835
Lukashev, N.V., Erzunov, D.A., Latyshev, G.V., Averin, A.D., and Beletskaya, I.P., Russ. J. Org. Chem., 2018, vol. 54, no. 1, p. 45. https://doi.org/10.1134/S1070428018010025
Bharadwaj, V., Park, J.E., Sahoo, S.K., and Choi, H.-J., ChemistrySelect, 2019, vol. 4, no. 36, p. 10895. https://doi.org/10.1002/slct.201902718
Tümay, S.O., J. Lumin., 2021, vol. 231, p. 117813. https://doi.org/10.1016/j.jlumin.2020.117813
Zhu, J.-H., Fan, X.-T., and Cao, Q.-Y., Inorg. Chim. Acta, 2016, vol. 451, p. 111. https://doi.org/10.1016/j.ica.2016.07.021
Sun, J., Xu, X., Yu, G., Li, W., and Shi, J., Tetrahedron, 2018, vol. 74, no. 9, p. 987. https://doi.org/10.1016/j.tet.2018.01.013
del Carmen González, M., Otón, F., Espinosa, A., Tárraga, A., and Molina, P., Org. Biomol. Chem., 2015, vol. 13, no. 5, p. 1429. https://doi.org/10.1039/c4ob02135e
Ghosh, K., Kar, D., Joardar, S., Samadder, A., and Khuda-Bukhsh, A.R., RSC Adv., 2014, vol. 4, no. 23, p. 11590. https://doi.org/10.1039/c3ra45018j
Tümay, S.O. and Yeşilot, S.J., Photochem. Photobiol. (A), 2019, vol. 372, p. 156. https://doi.org/10.1016/j.jphotochem.2018.12.012
Götzke, L., Schaper, G., März, J., Kaden, P., Huittinen, N., Stumpf, T., Kammerlander, K.K.K., Brunner, E., Hahn, P., Mehnert, A., Kersting, B., Henle, T., Lindoy, L.F., Zanoni, G., and Weigand, J.J., Coord. Chem. Rev., 2019, vol. 386, p. 267. https://doi.org/10.1016/j.ccr.2019.01.006
Pawara, S.V., Upadhyaya, P.K., Kumbhara, N., Buradea, S., Patilb, R., and Dhavalea, D.D., Carbohydr. Res., 2019, vol. 485, p. 107815. https://doi.org/10.1016/j.carres.2019.107815
Harit, T., Bellaouchi, R., Rokni, Y., Riahi, A., Malek, F., and Asehraou, A., Chem. Biodiversity, 2017, vol. 14, no. 12, p. e1700351. https://doi.org/10.1002/cbdv.201700351
Thota, B.N.S., Savyasachi, A.J., Lukashev, N., Beletskaya, I., and Maitra, U., Eur. J. Org. Chem., 2014, vol. 7, p. 1406. https://doi.org/10.1002/ejoc.201301443
Schweinfurth, D., Demeshko, S., Hohloch, S., Steinmetz, M., Brandenburg, J.G., Dechert, S., Meyer, F., Grimme, S., and Sarkar, B., Inorg. Chem., 2014, vol. 53, no. 16, p. 8203. https://doi.org/10.1021/ic500264k
Hagiwara, H., Minoura, R., Okada, S., and Sunatsuki, Y., Chem. Lett., 2014, vol. 43, no. 6, p. 950. https://doi.org/10.1246/cl.140133
Hapuarachchige, S. and Artemov, D., Top Magn. Reson. Imaging., 2016, vol. 25, no. 5, p. 205. https://doi.org/10.1097/RMR.0000000000000099
Hohloch, S., Deibel, N., Schweinfurth, D., Frey, W., and Sarkar, B., Eur. J. Inorg. Chem., 2014, vol. 2014, no. 12, p. 2131. https://doi.org/10.1002/ejic.201301339
Schweinfurth, D., Demeshko, S., Khusniyarov, M.M., Dechert, S., Gurram, V., Buchmeiser, M.R., Meyer, F., and Sarkar, B., Inorg. Chem., 2012, vol. 51, no. 14, p. 7592. https://doi.org/10.1021/ic300392e
Weisser, F., Stevens, H., Klein, J., van der Meer, M., Hohloch, S., and Sarkar, B., Chem. Eur. J., 2015, vol. 21, no. 24, p. 8926. https://doi.org/10.1002/chem.201406441
Baschieri, A., Mazzanti, A., Stagni, S., and Sambri, L., Eur. J. Inorg. Chem., 2013, vol. 2013, no. 13, p. 2432. https://doi.org/10.1002/ejic.201201361
Kudryavtsev, I.Y., Bykhovskaya, O.V., Matveeva, A.G., Baulina, T.V., Pasechnik, M.P., Matveev, S.V., Vologzhanina, A.V., Turanov, A.N., Karandashev, V.K., and Brel, V.K., Monatsh. Chem., 2020, vol. 151, no. 11, p. 1705. https://doi.org/10.1007/s00706-020-02702-6
Matveeva, A.G., Baulina, T.V., Kudryavtsev, I.Yu., Pasechnik, M.P., Aysin, R.R., Bykhovskaya, O.V., Godovikova, M.I., Matveev, S.V., Turanov, A.N., Karandashev, V.K., and Brel, V.K., Russ. J. Gen. Chem., 2020, vol. 90, no. 12, p. 2338. https://doi.org/10.1134/S107036322012018X
Matveeva, A.G., Bykhovskaya, O.V., Pasechnik, M.P., Vologzhanina, A.V., Aysin, R.R., Matveev, S.V., Godovikov, I.A., Kudryavtsev, I.Y., Baulina, T.V., and Brel, V.K., Mendeleev Commun., 2022, vol. 32, no. 5, p. 588. https://doi.org/10.1016/j.mencom.2022.09.006
Mitsunobu, O. and Yamada, Y., Bull. Chem. Soc. Japan, 1967, vol. 40, no. 10, p. 2380. https://doi.org/10.1246/bcsj.40.2380
Mitsunobu, O., Synthesis, 1981, no. 1, p. 1. https://doi.org/10.1055/s-1981-29317
Hughes, D.L., Org. React., 1992, vol. 42, p. 335. https://doi.org/10.1002/0471264180.or042.02
Zhai, R.L., Xue, Y.S., Liang, T., Mi J., J., and Xu, Z., J. Org. Chem., 2018, Vol. 83, p. 10051. https://doi.org/10.1021/acs.joc.8b01388
Kudryavtsev, I.Yu., Baulina, T.V., Khrustalev, V.N., Petrovskii, P.V., Pasechnik, M.P., and Nifant’ev, E.E., Doklady Chem., 2013, vol. 448, no. 2, p. 55. https://doi.org/10.1134/S0012500813020092
März, M., Chudoba, J., Kohout, M., and Cibulka, R., Org. Biomol. Chem., 2017, vol. 15, no. 9, p. 1970. https://doi.org/10.1039/c6ob02770a
Tornøe, C.W., Christensen, C., and Meldal, M., J. Org. Chem., 2002, vol. 67, no. 9, p. 3057. https://doi.org/10.1021/jo011148j
Dai, Z.-C., Chen, Y.-F., Zhang, M., Li, S.-K., Yang, T.-T., Shen, L., Wang, J.-X., Qian, S.-S., Zhu, H.-L., and Ye, Y.-H., Org. Biomol. Chem., 2015, vol. 13, no. 2, p. 477. https://doi.org/10.1039/C40B01758G
Matveeva, A.G., Vologzhanina, A.V., Pasechnik, M.P., Aysin, R.R., Matveev, S.V., Zubavichus, Y.V., Artyushin, O.I., Sharova, E.V., Godovikov, I.A., and Brel, V.K., Polyhedron, 2022, vol. 215, p. 115680. https://doi.org/10.1016/j.poly.2022.115680
Bykhovskaya, O.V., Matveeva, A.G., Pasechnik, M.P., Vologzhanina, A.V., Matveev, S.V., Kudryavtsev, I.Yu., Baulina, T.V., and Brel, V.K., Russ. J. Gen. Chem., 2019, vol. 89, no. 12, p. 2400. https://doi.org/10.1134/S1070363219120120
Baulina, T.V., Pasechnik, M.P., Kudryavtsev, I.Yu., Bykhovskaya, O.V., Sukat, G.Ya., Smol’yakov, A.F., Anikina, L.V., and Brel, V.K., J. Mol. Struct., 2020, vol. 1217, p. 128324. https://doi.org/10.1016/j.molstruc.2020.128324
Matveeva, A.G., Kudryavtsev, I.Yu., Pasechnik, M.P., Vologzhanina, A.V., Baulina, T.V., Vavina, A.V., Sukat, G.Ya., Matveev, S.V., Godovikov, I.A., Turanov, A.N., Karandashev, V.K., and Brel, V.K., Polyhedron, 2018, vol. 142, p. 71. https://doi.org/10.1016/j.poly.2017.12.025
Armarego, W.L.F. and Chai, C.L.L., Purification of Laboratory Chemicals, New York: Elsevier, 2009. https://doi.org/10.1134/S0044460X1809024X
Gel’man, N.E., Terent’eva, E.A., Shanina, T.M., and Kiparenko, L.M., Metody kolichestvennogo organicheskogo elementnogo mikroanaliza (Methods for Quantitative Organic Elemental Microanalysis), M.: Himija, 1987, p. 296.
SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
Sheldrick, G.M., Acta Crystalogr. (A), 2015, vol. 71, no. 1, p. 3. https://doi.org/10.1107/S2053273314026370
Sheldrick, G.M., Acta Crystallogr. (C), 2015, vol. 71, no. 1, p. 3. https://doi.org/10.1107/S2053229614024218
Funding
This study was financially supported by the Russian Science Foundation (grant no. 20-13-00329). Elemental analysis, registration of NMR, IR, and Raman spectra, and X-ray diffraction analysis were supported by the Ministry of Science and Higher Education of the Russian Federation and performed using the research equipment of the Center for Study of Molecular Structure, Institute of Organoelement Compounds, RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
V. K. Brel is a member of Editorial Board of the Russian Journal of General Chemistry. Other authors declare that they have no conflicts of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
Bykhovskaya, O.V., Kudryavtsev, I.Y., Baulina, T.V. et al. Unsymmetrical Tripodal Phosphine Oxide with Triazole Groups: Synthesis and Molecular Structure. Russ J Gen Chem 92, 1420–1429 (2022). https://doi.org/10.1134/S1070363222080084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222080084