Abstract
The novel compounds M2+Zr(SO4)3 with M = Mg, Mn, Co, Ni, Zn, and Cd as well as (Fe3+,2+,Zr)2(SO4)3 were synthesized at mild hydrothermal conditions (Teflon-lined stainless steel vessels, 220 °C) from the mixtures of Zr2O2(CO3)(OH)2, the respective M2+(SO4)·nH2O hydrated salts, H2SO4 and a minor amount of water. Crystals up to several tenths of a mm in size were obtained within a few days and studied at 200 K by single-crystal X-ray diffraction techniques. All these compounds belong to the structure type of monoclinic Fe2(SO4)3; they are either isotypic in space group P21/n (No. 14), Z = 4, i.e. M2+Zr(SO4)3 with M = Mn, Co, Ni, Zn, and Cd as well as the mixed valence sulfate (Fe3+,2+,Zr)2(SO4)3 or in the case of MgZr(SO4)3, closely related but with a larger unit cell, in space group Pc and Z = 8. The framework of the monoclinic Fe2(SO4)3 structure is characterized by two types of isolated Fe3+O6 octahedra, corner-linked with three types of sulfate groups. In the isotypic M2+Zr(SO4)3 series, the Fe3+ atom on the Fe(1) position is substituted by Zr4+ while M2+ ions occupy the Fe(2) site in the ferric sulfate structure type. Mean cation-oxygen bond lengths (S[4]: 1.462–1.472 Å; Zr[6]: 2.053–2.060 Å as well as M2+–O distances) are generally rather short, but still within the range reported in literature.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We recently have initiated a study on the crystal chemistry of novel zirconium oxysalts synthesized under mild hydrothermal conditions. In part I the compounds Zr(SeO4)2·H2O and Zr(SeO4)2·4H2O have been described [1] while part II characterized Zr(SeO3)(SeO4), Zr4(SeO3)(SeO4)7, and Zr3(SeO3)(SeO4)5·2H2O [2].
Part III now deals with a new group of isotypic or closely related sulfates of the general formula M2+Zr(SO4)3 with M = Mg, Mn, Co, Ni, Zn, and Cd, as well as (Fe3+,2+,Zr)2(SO4)3. These compounds will be referred to as MZr or e.g. abbreviated as MgZr for MgZr(SO4)3 throughout the further text. Evaluation of unit cell parameters for (Fe3+,2+,Zr)2(SO4)3 and MZr (M = Mn, Co, Ni, Zn, Cd) suggested isotypy with monoclinic Fe2(SO4)3.
Anhydrous ferric sulfate is known to occur in two modifications, a rhombohedral one (mikasaite) in space group R\(\overline{3}\) [3, 4], and a monoclinic one in space group P21/n [5,6,7], the latter being the type structure of a series of compounds. Further representatives described in space group P21/n (No. 14) with Z = 4 are e.g. In2(SO4)3 [8], Sc2(SeO4)3 [9], Yb2(SeO4)3 [10], Fe2(SeO4)3 [11], Al2(WO4)3 [12], Sb2(PO4)3 [13], and V2(PO4)3 [14].
For the compound MgZr a larger monoclinic unit cell with Z = 8 was obtained. This phase may be derived from the AlFe(MoO4)3 structure type (space group P21/a (No. 14) with Z = 8 [15]), but with reduced symmetry (space group Pc (No. 7)). Several other molybdates belong to the AlFe(MoO4)3 type, among them In2(MoO4)3 [16], Cr2(MoO4)3 [17], Fe2(MoO4)3 [18], and Al2(MoO4)3 [19], further also In2(WO4)3 [20].
The present paper aims to elucidate the new class of MZr compounds, especially their structure relationships to Fe2(SO4)3 and AlFe(MoO4)3. Our report on zirconium oxysalts will be continued (part IV), describing Zr(SeO3)2 as well as structure refinements of Zr2(OH)2(XO4)3·4H2O (X = S, Se) and a new modification of Zr(SO4)2·4H2O.
Results and discussion
All compounds presented belong or are related to the structure type of monoclinic Fe2(SO4)3; they are either isotypic in space group P21/n (No. 14), Z = 4, i.e. MZr with M = Mn, Co, Ni, Zn, and Cd as well as the mixed valence sulfate (Fe3+,2+,Zr)2(SO4)3, or, in the case of MgZr, closely related but with a larger unit cell and space group Pc, Z = 8.
Selected individual and mean bond lengths and bond valence strengths for the MZr compounds as well as for (Fe3+,2+,Zr)2(SO4)3 and Fe2(SO4)3 are listed in Tables 1, 2 (all measurements at 200 K).
Structure details for Fe2(SO4)3, i.e. bond lengths, polyhedral distortion and the specific arrangement of the framework show no significant differences with the earlier results obtained at room temperature. The cell volume given in [7] is 823.6 Å3, as compared to our data 819.4(1) Å3, measured at 200 K. A study at ~ 4 K by neutron diffraction techniques [21] reports a cell volume of 788.8 Å3.
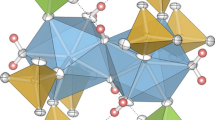
The framework of the monoclinic type structure Fe2(SO4)3 (Fig. 1a) is characterized by two sites of isolated Fe3+O6 octahedra, corner-linked with three types of sulfate groups. The mean Fe–O bond lengths of the Fe(1) site are somewhat shorter (1.978 Å) than those of the Fe(2) position (1.993 Å). In the isotypic MZr series (Fig. 1b), M2+ and Zr4+ were found to be strictly ordered (within the limits of error), with Zr4+ occupying the Fe(1) and M2+ the Fe(2) position of the ferric sulfate structure type for MZr members with M2+ = Mn, Co, Ni, Zn and Cd. The mean [6]Zr–O distance is generally shorter (~ 2.06 Å), as compared to the respective [6]M–O bond lengths, which range from 2.06 Å (Ni) up to 2.26 Å (Cd).
Partial cation disorder was found in the mixed-valence sulfate (Fe3+,2+,Zr)2(SO4)3, which can be expressed as (Fe3+2−2x, Fe2+x, Zrx)(SO4)3 with x ~ 0.21, assuming full site occupancies. Also in this case, zirconium is preferentially substituting the Fe(1) site with 16% while the Fe(2) position shows 5% Zr. A representative with x = 1, i.e. an ordered FeZr phase containing only ferrous iron, could not be synthesized up to now.
In monoclinic Fe2(SO4)3, each oxygen atom is bound to one S6+ and one Fe3+ cation. Individual S–O distances for the three different sulfate groups show minor bond length distortions only. Contrary, in MZr compounds with M = Mn, Co, Ni, Zn, and Cd the S–O distances are clearly separated into two groups: oxygen atoms shared with ZrO6 octahedra have S–O distances of about 1.49 Å, while those belonging to M2+O6 polyhedra show considerably shorter S–O bond lengths (~ 1.44 Å). This can be explained by the bond strength contributions of the tetravalent zirconium in comparison to the respective divalent M cation. In the more complex structure type of MgZr discussed later, an even more pronounced splitting of the corresponding S–O distances is evident. Comparable bond length distortions of sulfate groups are commonly observed, e.g. in ZnSO4 [23] or α-MgSO4 [24].
The S–O–Fe3+ bond angles in Fe2(SO4)3 vary between 135 and 160°. In general, the respective values for MZr compounds exhibit similar trends even though they are somewhat modified by the individual substitution of ferric iron by M2+ and Zr4+, respectively.
The crystal structure of MgZr (Fig. 2b) is closely related with that of the other MZr members (Fig. 2a), as well as with compounds which belong to the AlFe(MoO4)3 type (Fig. 2c). This is evident from the unit cell transformations, illustrated in Fig. 3.
Comparison of corresponding sections of the crystal structures of a NiZr(SO4)3 (as representative of the P21/n-type MZr compounds), b MgZr(SO4)3, and c) AlFe(MoO4)3 in projections along the crystallographic b-axes. Note the different distribution of M2+ vs. Zr in the Ni- compared to the Mg-compound, whereas in AlFe(MoO4)3, all octahedra are equally occupied by Al0.5Fe0.5 [8]
By the transformation (½ 0 ½/0 1 0/− ½ 0 ½), the lattice parameters of MgZr [a = 14.902 Å, b = 8.759 Å, c = 14.896 Å, β = 108.70, V = 1841.7 Å3, Z = 8, space group Pc] change to a monoclinic subcell with a = 8.684 Å, b = 8.759 Å, c = 12.107 Å, β = 90.024°, V = 920.84 Å3, Z = 4, resembling the unit cells of the other MZr compounds (see Table 3). By transformation via (− 1 0 − 1/0 1 0/1 0 0), the unit cell of AlFe(MoO4)3 [a = 15.509 Å, b = 9.132 Å, c = 18.021 Å, β = 125.31°, V = 2082.9 Å3, Z = 8, space group P21/a] turns into [a = 15.564 Å, b = 9.132 Å, c = 15.509 Å, β = 109.104°, V = 2082.9 Å3, Z = 8, space group setting P21/c] revealing its relationship to MgZr. The unit cell parameters of MgZr can also be transformed to a close to orthorhombic C-centred cell [a = 17.367 Å, b = 24.214 Å, c = 8.759 Å, α = β = 90°, γ = 90.024°, V = 3683.3 Å3, Z = 16] by the transformation (1 0 1/− 1 0 1/0 1 0), but no reliable orthorhombic structure model could be refined.
Within the investigated series, the lattice parameters shown in Fig. 4a (including the transformed values for MgZr) seem to deviate from a linear relationship with the M2+ cationic radii, whereas the mean M–O bond lengths (Fig. 4b) obviously behave according to Vegard’s rule. This might be explained by the flexibility of this structure type in terms of angular variation at the bridging polyhedral oxygen corners, thus enabling a partial compensation of the MO6 polyhedral volume increase.
Behaviour of a the lattice parameters (with second order regression lines), and b the overall mean M–O bond lengths with increasing r(M) [29] along the MZr series. Note: Fe* = Fe2+0.21Fe3+0.74Zr0.05
On the other hand, the Fe2(SO4)3 structure type does not seem to be flexible enough to allow the accommodation of Mg at the M site. Investigations of kieserite-, blödite-, and Tutton-type sulfates [25,26,27,28] indicate that the absence of 3d orbitals in Mg compared to the M2+ transition metal cations strongly influences its crystal chemical behaviour, especially concerning a tendency towards comparatively larger Mg–O–S angles at the bridging oxygen atoms. As a consequence, the octahedral Mg/Zr distribution and the structure type changes. In fact, the overall mean M–O–S and Zr–O–S angles in the MZr compounds (without Mg) are 142.5° and 149.7°, respectively, whereas respective angles in the MgZr compound are 150.5° and 154.2°.
Conclusion
A series of novel M2+Zr(SO4)3 compounds with M = Mg, Mn, Co, Ni, Zn, and Cd as well as (Fe3+,2+,Zr)2(SO4)3 were obtained at mild hydrothermal conditions by the reaction of Zr2O2(CO3)(OH)2 with M2+(SO4)·nH2O, H2SO4 and H2O. All these compounds are either isotypic or at least related to the structure type of monoclinic Fe2(SO4)3 in space group P21/n, Z = 4, where two types of isolated Fe3+O6 octahedra occur, corner-linked with three types of sulfate groups to a framework structure. In the isotypic series M2+Zr(SO4)3 with M = Mn, Co, Ni, Zn, and Cd, Zr4+ substitutes Fe3+ on the Fe(1) position, while M2+ ions occupy the Fe(2) site of the ferric sulfate structure type. In the mixed valence sulfate (Fe3+2-2x, Fe2+x, Zrx)(SO4)3 with x ~ 0.21, zirconium is also preferentially incorporated in the Fe(1) position. The structure of MgZr(SO4)3 is closely related, but with a larger unit cell and space group Pc, Z = 8. Compared to the M2+Zr transition metal compounds, the absence of 3d orbitals in case of the Mg cation seems to play a decisive role for its differing structural behaviour.
Experimental
Synthesis
The investigated zirconium sulfates were obtained by mild hydrothermal synthesis from mixtures of ~ 0.03 g Zr2O2(CO3)(OH)2, 0.08 g of the respective M2+(SO4)·nH2O hydrated salts, 0.1 g 93% H2SO4, and minor contents of water (0.5–1 cm3). These reagents were filled in Teflon-lined steel vessels with a capacity of a few cm3, heated up to 220 °C within several hours, kept at this temperature for a period of one week, and finally cooled down to room temperature within 24 h. Various crystals of up to 0.4 mm had formed, which were separated from the remaining liquid, washed in water and ethanol, dried, and embedded in silicone grease.
X-ray diffraction data and crystal structure determination
Small fragments of the title compounds with homogeneous extinction under crossed polars were used for single crystal X-ray data collections at 200 K. MgZr, MnZr, CoZr, and NiZr were measured on an Enraf–Nonius Kappa CCD diffractometer (sealed tube, equipped with a graphite monochromator + monocapillary optics using Mo-Kα radiation and an Oxford Cryosystems Cryostream 600 device). The other compounds were investigated on a Bruker APEXII diffractometer equipped with a CCD area detector, an Incoatec Microfocus Source IµS (30 W, multilayer mirror, Mo-Kα), and an Oxford Cryosystems Cryostream 800 Plus LT device. Several sets of phi- and omega-scans with 2° scan width were measured at a crystal-to-detector distance of 30 or 35 mm, respectively, up to 80° 2θ full sphere. The absorption was corrected by the evaluation of multi-scans.
The crystal structure of MgZr was solved by direct methods (Shelxs-97 [30]). For all other compounds, the atomic coordinates of monoclinic ferric sulfate given in [7] were used as starting parameters and refined by full-matrix least-squares techniques (Shelxl-97 [30]). The distribution of M2+ and Zr4+ over the two cation sites Fe(1) and Fe(2), as labelled in the type structure of Fe2(SO4)3, was checked by site-occupancy refinement. In (Fe3+,2+,Zr)2(SO4)3 minor amounts of zirconium were refined at the Fe(1) and Fe(2) sites with contents of 16% and 5%, respectively. Selected crystal parameters as well as a summary on the data collections and structure refinements are given in Table 3.
Final atomic positions and important interatomic bond distances are listed in Tables 1, 2, 4, 5. Crystallographic data have also been deposited and may be obtained at https://icsd.fiz-karlsruhe.de by referencing the CSD-numbers for M2+Zr(SO4)3 compounds with M = Mg: 1939563, Mn: 1939562, Co: 1939565, Ni: 1939564, Zn: 1939566, and Cd: 1939560, as well as for (Fe3+,2+,Zr)2(SO4)3: 1939567 and Fe2(SO4)3: 1939561.
Change history
26 October 2019
The original version of this article unfortunately contained a mistake in Table 3. The corrected Table 3 is given below. The original article has been corrected.
References
Giester G, Wildner M (2018) Monatsh Chem 149:1321
Wildner M, Giester G (2019) Monatsh Chem 150:593
Masse R, Guitel JC, Perret R (1973) Bull Soc Fr Mineral Cristallogr 96:346
Christidis PC, Rentzeperis PJ (1976) Z Kristallogr, Kristallgeom, Kristallphys. Kristallchem 144:341
Moore PB, Araki T (1974) Neues Jahrb Mineral Abh 121:208
Christidis PC, Rentzeperis PJ (1975) Z Kristallogr, Kristallgeom, Kristallphys. Kristallchem 141:233
Christidis PC, Rentzeperis PJ, Kirfel A, Will G (1983) Z Kristallogr 164:219
Krause M, Gruehn R (1995) Z Kristallogr 210:427
Valkonen J (1978) Acta Crystallogr B 34:1957
Iskhakova LD, Ovanisyan SM (1995) Russ J Inorg Chem 40:1702
Giester G, Wildner M (1991) Monatsh Chem 122:617
Hanuza J, Maczka M, Hermanowicz K, Andruszkiewicz M, Pietraszko A, Strek W, Dereń P (1993) J Sol State Chem 105:49
Jouanneaux A, Verbaere A, Guyomard D, Piffard Y, Oyetola S, Fitch AN (1991) Eur J Solid State Inorg Chem 28:755
Yin S-C, Grondey H, Strobel P, Anne M, Nazar LF (2003) J Am Chem Soc 125:10402
Harrison WTA, Cheetham AK (1989) Acta Crystallogr C 45:178
Plyasova LM, Klevtsova RF, Borisov SV, Kefeli LM (1968) Kristallografiya 13:38
Battle PD, Cheetham AK, Harrison WTA, Faber J Jr (1985) J Sol State Chem 58:221
Chen H-Y (1979) Mater Res Bull 14:1583
Harrison WTA, Cheetham AK, Faber J Jr (1988) J Sol State Chem 76:328
Richard AP, Edwards DD (2004) J Sol State Chem 177:2740
Long G-J, Longworth G, Battle PD, Cheetham AK, Thundathil RV, Beveridge D (1979) Inorg Chem 18:624
Brese NE, O’Keeffe M (1991) Acta Crystallogr B 47:192
Wildner M, Giester G (1988) Mineral Petrol 39:201
Benmakhlouf A, Errandonea D, Bouchenafa M, Maabed S, Bouhemadoud A, Bentabete A (2017) Dalton Trans 46:5058
Wildner M, Giester G (1991) Neues Jahrb Mineral Monatsh 1991:296
Stoilova D, Wildner M (2004) J Mol Struct 706:57
Bechtold A, Wildner M (2016) Eur J Mineral 28:43
Bosi F, Belardi G, Ballirano P (2009) Am Mineral 94:74
Shannon RD (1976) Acta Crystallogr A 32:751
Sheldrick GM (2008) Acta Crystallogr A 64:112
Acknowledgements
Open access funding provided by University of Vienna. This study was financially supported by grants IS526001 and IP532010 of the University of Vienna. We are grateful to Prof. H. Effenberger for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Giester, G., Talla, D. & Wildner, M. Contributions to the stereochemistry of zirconium oxysalts—part III: syntheses and crystal structures of M2+Zr(SO4)3 with M = Mg, Mn, Co, Ni, Zn and Cd, and a note on (Fe3+,2+,Zr)2(SO4)3 and Fe2(SO4)3. Monatsh Chem 150, 1877–1892 (2019). https://doi.org/10.1007/s00706-019-02496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02496-2