Abstract
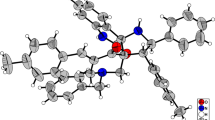
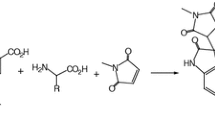
A diastereoselective practical route to functionalized spiropyrrolizidine-linked rhodanines has been developed via 1,3-dipolar cycloaddition reaction of alkylidenerhodanines with azomethine ylides, prepared in situ from l-proline and acetylenic esters, in EtOH at reflux. This protocol provided quick access to a range of structurally diverse target molecules in moderate-to-good yields. The structure of a typical product was established by X-ray crystallography.
Graphic abstract




Similar content being viewed by others
References
Mao ZY, Liu YW, Han P, Dong HQ, Si CM, Wei BG, Lin GQ (2018) Org Lett 20:1090
Li C, Zhang F (2018) ChemistrySelect 3:1815
Wang X, Li M, Yang Y, Guo M, Tang X, Wang G (2018) Adv Synth Catal 360:2151
Liu YY, Yu XY, Chen JR, Qiao MM, Qi X, Shi DQ, Xiao WJ (2017) Angew Chem Int Ed 56:9527
Alizadeh A, Rezvanian A, Zhu AL (2012) J Org Chem 77:4385
Chen Z, Wu J (2010) Org Lett 12:4856
Hall DG, Rybak T, Verdelet T (2016) Acc Chem Res 49:2489
Isambert N, Duque MDMS, Plaquevent JC, Genisson Y, Rodriguez J, Constantieux T (2011) Chem Soc Rev 40:1347
Yu L, Somfai P (2019) RSC Adv 9:2799
Banerjee B (2017) Ultrason Sonochem 35:15
Anand D, Yadav PK, Patel OPS, Parmar N, Maurya RK, Vishwakarma P, Raju KSR, Taneja I, Wahajuddin M, Kar S, Yadav PP (2017) J Med Chem 60:1041
Sakhuja R, Pericherla K, Bajaj K, Khungar B, Kumar A (2016) Synthesis 48:4305
Scaggs WR, Scaggs TD, Snaddon TN (2019) Org Biomol Chem 17:1787
Aboelmagd M, Elokely K, Zaki MA, Said A, Haggag EG, Ross SA (2018) Med Chem Res 27:1066
Parhizkar A, Khosropour AR, Mohammadpoor-Baltork I, Parhizkar E, Amiri Rudbari HA (2017) Tetrahedron 73:1397
Dandia A, Jain AK, Laxkar AK (2013) RSC Adv 3:8422
Karthikeyan SV, Bala BD, Raja VPA, Perumal S, Yogeeswari P, Sriram D (2010) Bioorg Med Chem Lett 20:350
Yu B, Yu DQ, Liu HM (2015) Eur J Med Chem 97:673
Schramm S, Köhler N, Rozhon W (2019) Molecules 24:498
Tkachuk AV, Kurbatov SV, Burov ON, Kletskii ME, Morozov PG, Minkin VI (2014) Chem Heterocycl Compd 50:26
Thangamani A (2010) Eur J Med Chem 45:6120
Wen R, Cen L, Ma Y, Wang J, Zhu S (2018) Tetrahedron Lett 59:1686
Li YX, Shimada Y, Sato K, Kato A, Zhang W, Jia YM, Fleet GWJ, Xiao M, Yu CY (2015) Org Lett 17:716
Manjappa KB, Peng YT, Jhang WF, Yang DY (2016) Tetrahedron 72:853
Bradner WT (2001) Cancer Treat Rev 27:35
Sobenina LN, Tomilin DN, Sagitova EF, Ushakov IA, Trofimov BA (2017) Org Lett 19:1586
Hao F, Reddy AR, Zhou CY, Che CM (2018) Adv Synth Catal 360:1433
Li J, Wang J, Xu Z, Zhu S (2014) ACS Comb Sci 16:506
Babu ARS, Raghunathan R (2008) Tetrahedron Lett 49:4618
Song YX, Du DM (2018) J Org Chem 83:9278
Li P, Zhang W, Jiang H, Li Y, Dong C, Chen H, Zhang K, Du Z (2018) Med Chem Commun 9:1194
Tejchman W, Korona-Glowniak I, Malm A, Zylewski M, Suder P (2017) Med Chem Res 26:1316
Slepikas L, Chiriano G, Perozzo R, Tardy S, Kranjc-Pietrucci A, Patthey-Vuadens O, Ouertatani-Sakouhi H, Kicka S, Harrison CF, Scrignari T, Perron K, Hilbi H, Soldati T, Cosson P, Tarasevicius E, Scapozza L (2016) J Med Chem 59:10917
El-Sonbati AZ, Diab MA, El-Bindary AA, Mohamed GG, Morgan ShM (2015) Inorg Chim Acta 430:96
Subhedar DD, Shiakh MH, Shingate BB, Nawale L, Sarkar D, Khedkar VM, Khan FAK, Sangshetti JN (2017) Eur J Med Chem 125:385
Stawoska I, Tejchman W, Mazuryk O, Lyčka A, Nowak-Sliwinska P, Żesławska E, Nitek W, Kania A (2017) J Heterocycl Chem 54:2889
Wang X, Lin H, Xu S, Jin Y, Zhang R (2018) Medicine 97:9828
Rostamnia S, Lamei K (2011) Synthesis 19:3080
Chen L, Sun J, Xie J, Gong H, Yun CG (2016) Org Biomol Chem 14:6497
Sun J, Chen L, Gong H, Yun CG (2015) Org Biomol Chem 13:5905
Yung F, Sun J, Gao H, Yun CG (2015) RSC Adv 5:32786
Pandey G, Banerjee P, Gadre SR (2006) Chem Rev 106:4484
Coldham I, Hufton R (2005) Chem Rev 105:2765
Acknowledgements
We would like to thank the Research Council of Tarbiat Modares University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yavari, I., Sheikhi, S., Taheri, Z. et al. A diastereoselective synthesis of functionalized spiropyrrolizidine-linked rhodanines. Monatsh Chem 150, 1825–1831 (2019). https://doi.org/10.1007/s00706-019-02485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02485-5