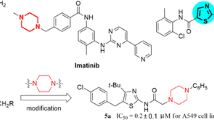
Abstract
A series of new 2-(3-aminophenyl)-benzothiazole derivatives were synthesized and evaluated for their in vitro antiproliferative activity against various human cancer cell lines including A549, HeLa, HepG2, MCF-7, MV4-11, and DB. Among the tested compounds, N-[3-(benzo[d]thiazol-2-yl)phenyl]nicotinamide displayed significantly improved antiproliferative activity toward A549 and MV4-11 cells with IC50 values of 5.42 ± 1.33 and 7.51 ± 0.98 μM, respectively, much stronger than the hit 3-(benzo[d]thiazol-2-yl)-N-(4-bromobenzyl)aniline. Furthermore, flow cytometric analysis indicated that N-[3-(benzo[d]thiazol-2-yl)phenyl]nicotinamide induced A549 cell apoptosis with cell cycle arrest at G1 phase in a concentration-dependent manner.
Graphical abstract




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) CA Cancer J Clin 61:69
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016) Lancet 388:1545
World Cancer Report (2014) World Health Organization. Accessed 3 Mar 2018
Zhang J, Yang F, Qiao Z, Zhu M, Zhou H (2016) Bioorg Med Chem Lett 26:5797
Tantawy MA, Nafie MS, Elmegeed GA, Ali IAI (2017) Bioorg Chem 73:128
Rai A, Singh AK, Raj V, Saha S (2018) Mini Rev Med Chem 18:42
Akhtar MJ, Yar MS, Khan AA, Ali Z, Haider MR (2017) Mini Rev Med Chem 17:1602
Rouf A, Tanyeli C (2015) Eur J Med Chem 97:911
Mei WW, Ji SS, Xiao W, Wang XD, Jiang CS, Ma WQ, Zhang HY, Gong JX, Guo YW (2017) Monatsh Chem 148:1807
Thakkar SS, Thakor P, Ray A, Doshi H, Thakkar VR (2017) Bioorg Med Chem 25:5396
Bhat M, Belagali SL (2018) Future Med Chem 10:71
Cindrić M, Jambon S, Harej A, Depauw S, David-Cordonnier MH, Kraljević Pavelić S, Karminski-Zamola G, Hranjec M (2017) Eur J Med Chem 136:468
Al-Harthy T, Zoghaib WM, Stoll R (2018) Monatsh Chem 149:645
Kamal A, Syed MA, Mohammed SM (2015) Expert Opin Ther Pat 25:335
Capuyo R, Calabrò ML, Micale N, Schimmer AD, Ali M, Zappalà M, Grasso S (2015) Med Chem Res 21:2644
Hutchinson I, Chua MS, Browne HL, Trapani V, Bradshaw TD, Westwell AD, Stevens MF (2001) J Med Chem 44:1446
Zhang Y, Chakraborty M, Cerda-Smith CG, Bratton RN, Maurer NE, Senser EM, Novak M (2013) J Org Chem 78:6992
Chhabra M, Sinha S, Banerjee S, Paira P (2016) Bioorg Med Chem Lett 26:213
Ehlert J, Herz T, Krauss R, Kubbutat M, Lang M, Saeb W, Schaechtele C, Tasler S, Totzke F, Zirrgiebel U (2007) 2-Arylbenzothiazole analogs as kinase inhibitors and their preparation, pharmaceutical compositions and use in the treatment of kinase-mediated diseases. US patent 20070021446 ((2007) Chem Abstr 146:184430)
Ehlert J, Herz T, Krauss R, Kubbutat M, Martin L, Saeb W, Schaechtele C, Tasler S, Totzke F, Zirrgiebel U (2007) Preparation of 2-heteroarylaminophenylbenzothiazoles as anticancer drugs. European patent EP 1746096 A1 ((2007) Chem Abstr 146:184428)
Asahara H (2017) Proc Eng 174:1046
Mosmann T (1983) J Immunol Methods 65:55
Wang J, Sánchez-Roselló M, Aceña JL, del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H (2014) Chem Rev 114:2432
Shankaraiaha N, Nekkantia S, Brahmab UR, Kumara NP, Deshpandea N, Prasannaa D, Senwara KR, Lakshmib UJ (2017) Bioorg Med Chem 25:4805
Wade AR, Pawar HR, Biware MV, Chikate RC (2015) Green Chem 17:3879
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (No. 21672082), Shandong Key Development Project (No. 2016GSF201209), the Young Taishan Scholars Program (No. tsqn20161037), Natural Science Foundation of Shandong Province (Nos. ZR2017BH038, JQ201721), Shandong Talents Team Cultivation Plan of University Preponderant Discipline (No. 10027), and the Brazilian Government Agencies FAP/DF and CNPq.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Cheng, ZQ., Song, JL. et al. Synthesis and biological evaluation of 2-(3-aminophenyl)-benzothiazoles as antiproliferative and apoptosis-inducing agents. Monatsh Chem 149, 2093–2102 (2018). https://doi.org/10.1007/s00706-018-2274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2274-z