Abstract
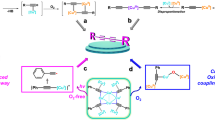
We have investigated the photo-induced reduction of Cu2+–Cu0 using benzil/triethylamine mixtures. The formation of elemental Cu is indicated by the appearance of its characteristic plasmon absorption peaks at 515 nm and 620 nm. Importantly, the nature of the counterion of the Cu2+ salt affects the reduction process. In the presence of Cl−, the reduction proceeds faster than with SO42-. Photo-induced electron transfer between excited benzil and triethylamine leads to the benzil radical anion, which acts as the reducing agent for Cu2+ and generates Cu0.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have experienced a vast growth in interest over the last years. They have been utilized in fields like nonlinear optics and electric conduction [1,2,3]. Metallic nanoparticles have gained attention because of their remarkable chemical properties, leading to applications, e.g., for molecular imaging [4] or catalysis [5].
A key point for producing nanoparticles is the reduction of metal salts to elemental metals in a controlled way. Chemical, thermal, radiation-chemical, sonochemical, and photochemical methods have been followed in this context [6,7,8,9]. However, many of these approaches require expensive reagents, hazardous reaction conditions, and long reaction times combined with difficult isolation procedures [7, 10]. Photochemical methods offer a valuable access to metal reductions allowing temporal and spatial control [11,12,13,14,15,16,17,18]. In such procedures, organic radical anions, produced by photochemical reduction, act as mediators, reducing metal cations to elementary metals [16, 19].
A group of reagents often employed in photo-reductions of metal salts include ketones such as acetophenone [11], acetone [20], and benzophenone [21]. The use of ketones combined with hydrocarbons, alcohols, ethers, and amines has been reported [22, 23]. In such reactions, highly reactive ketyl radicals or ketyl radical anions are formed as intermediates. A substantial requirement for the reducing species is their oxidation potential, since it has to match the potential for the reduction of the metal cation. In terms of metals, the generation of Cu nanoparticles from Cu2+ salts has been of prominent interest because of the favorable availability of Cu salts and the activity of Cu as catalyst, in photovoltaics, electronics, and optics [24,25,26,27].
The aim of our investigation is to inspect if benzil (1,2-diphenylethane-1,2-dione, 1) can be utilized for the photo-reduction of Cu2+ salts. Benzil is one of the most common (and low cost) diketones and its photochemistry has been well characterized [28, 29]; nevertheless, benzil has yet only seen limited use in photo-induced redox reactions with metal salts.
While the photo-reduction of ketones and diketones in the presence of donor systems, e.g., amines, and the intermediate formation of ketyl radicals are well understood, there are still remaining questions with regard to the mechanism of metal reduction. The mechanisms depicted in Scheme 1 have been suggested for the photo-reduction of metal salts. Here, either ketyl radicals [11, 12, 17, 21] or the ketyl radical anions [30] act as electron donors.

In this publication, we report on the reaction of photo-excited benzil (1) and triethylamine (2) as a model donor with Cu2+ salts. We followed the reactions by steady-state photolysis (SSP), continuous-wave electron paramagnetic resonance (cw-EPR) spectroscopy, and laser-flash photolysis (LFP).
Results and discussion
Steady-state photolysis
We photolyzed (steady state) a solution of 1 and 2 in CH3CN containing either CuCl2 or CuSO4 and observed a new strong band centered at 515 nm and a weaker band at 620 nm (Fig. 1). They can be attributed to characteristic plasmon absorption bands of colloidal Cu [31,32,33,34,35]. It is well established that the plasmon absorption of elemental copper depends on the size of the aggregates formed [34,35,36]. Therefore, the two absorption bands are in line with an initial formation of small copper aggregates resulting in the band centered at 515 nm, whereas that at 620 nm points to the slower growth of bigger colloids. Control experiments with solutions of the mixtures 1/2, 1/CuCl2 (CuSO4), 2/CuCl2 (CuSO4), and singly CuCl2 or CuSO4 (see SI) substantiate these findings. None of these experiments yielded the bands at 515 and 620 nm upon photolysis. The solution 1/2 showed absorption spectra indicating the bleaching of the band attributed to parent benzil at 360 nm [37], whereas no spectral changes could be detected for the remaining controls. Accordingly, the copper salts are not decomposed in our irradiation experiments and are inert toward benzil in the absence of the amine and vice versa.
Remarkably, the rates for the reduction depend on the counterions of the copper salts (Fig. 2). For CuCl2, the bands attributed to the plasmon absorption grow in at a faster rate than for CuSO4. This is in line with the results of Pacioni et al. as well as Soares et al., who reported that chloride anions catalyze the disproportionation mediating the conversion of Cu+ to Cu0 [33, 37]. In addition, electrochemical studies have shown that catalytic amounts of Cl− accelerate the reduction of Cu2+ to Cu+ [38, 39, 40] (Scheme 2).

Continuous-wave electron paramagnetic resonance
When we observed the solutions of 1 in the presence of 2 in CH3CN by cw-EPR under continuous irradiation, we detected the characteristic EPR spectrum attributable to the radical anion of benzil (1·−). Simulations reveal (Fig. 3, Table 1) an excellent agreement with previously published data [41, 42]. The formation of 1·− indicates that upon irradiation, 1 undergoes excitation and intersystem crossing to the triplet state 1* [43] and is subsequently reduced by 2, as indicated in Scheme 3. This is in line with previous studies showing that also for other aromatic ketones such as benzophenone, the corresponding radical anion was observed in photolysis experiments in the presence of alcohols [44] and amines [45].

The radical cation of 2·+, formed together with 1·−, is not detected in the EPR spectrum. It is well established that radical cations of amines undergo follow-up reactions, leading to both diamagnetic and paramagnetic species [23, 37, 46, 47] (Scheme 4). Besides parent 2, the α-aminoalkyl radical 4 may serve as an electron-donating species [17, 37, 48]. Therefore, an additional reduction of Cu2+ by 4 should be considered when discussing the redox reactions in this system [48]. However, it was shown that 2·+ can undergo rapid follow-up reactions leading to the formation of diamagnetic species [37, 49, 50]. Additionally, product analysis by 1H NMR after irradiation (see Supporting Information) reveals formation of N,N-diethylethenamine (5), further rationalizing that the electron transfer from triethylamine-derived radicals only plays a minor role in this system.

Laser-flash photolysis
To evaluate the role of 2 for the reduction of Cu2+ to Cu0 at a short (ns) time scale, we carried out laser-flash photolysis (LFP) experiments. Figure 4 shows the transient absorption spectra of 1/2 in CH3CN and the corresponding reference measurements, in which 2 was omitted. Photolysis of 1 in CH3CN yields two distinct peaks at 350 nm and at 480 nm, which are both attributable to 1* [31, 35, 43]. Upon addition of 2 to the solution, significant changes in the spectrum occur: The absorptions centered at 480 and 350 nm disappear, while two new, broad bands centered at 360 and 580 nm appear; they are assigned to 1·− [51, 52, 53]. This indicates a fast electron transfer reaction of 1* with 2, leading to the formation of 1·− and 2·+, respectively [37, 54].
Conclusions
Our spectroscopic investigation shows that benzil acts as an efficient photo-reducing agent for copper salts in the presence of an amine donor. The efficiency and the rate of the photo-reduction are markedly influenced by the counterions of the copper salts. We have demonstrated the intermediate formation of the benzil radical anion by cw-EPR and LFP. From our experiments, we conclude that 1·−, formed upon photolysis in the presence of 2, is highly redox active and can reduce Cu2+ to Cu0. In addition, no indication that the corresponding radical cation of 2 takes part in the reduction of Cu2+ to Cu0 could be found. This is presumably due to the fast follow-up reaction of 2+·, leading to the formation of diamagnetic products that are not redox active.
In future work, this cost-effective and simple approach for the photo-induced reduction of Cu2+ to elemental copper could be used for the production of copper nanoparticles by employing different irradiation times as well as characterization methods such as transmission electron microscopy (TEM) to form nanoparticles with defined shape and size [16, 55, 56].
Experimental
Benzil (1, Fluka), triethylamine (2, Sigma-Aldrich), copper(II) sulfate (Roth), copper(II) chloride (Riedel-de Haën), and acetonitrilie (Riedel-de Haën) were obtained at the highest purity available and employed as received.
Steady-state photolysis
UV–Vis spectra were recorded on a fiber optic diode array spectrometer (J&M Analytik AG). Photolysis was conducted using a Hamamatsu Lightingcure LC4 (Hg–Xe lamp, 3500 mW/cm2, λmax = 365 nm). The concentrations of 1, CuSO4, and CuCl2 were 5 mM for all measurements. The concentration of 2 was 100 mM in all measurements. Polyvinylpyrrolidone (10 mg) was added to all samples to help solubilize the copper salts and precipitate the formed Cu0.
EPR spectroscopy
Cw-EPR spectra were recorded on a Bruker X-band spectrometer (EMX, 100 kHz field modulation) at room temperature with 0.025 mT field modulation amplitude. The signals correspond to the steady-state concentration of radicals accumulated in the flow system (0.4 mm quartz flat cell) under continuous irradiation The concentrations of 1 and 2 were 100 and 500 mM, respectively.
Laser-flash photolysis
LFP experiments were carried out with an LKS80 spectrometer (Applied Photophysics). The excitation of the samples was carried out with the light of a frequency triplet Spitlight Compact 100 (Innolas) Nd:YAG laser at 355 nm (8 ns pulse duration; 10 mJ/pulse energy). The concentration of 1 in solution was adjusted to achieve absorbance of around 0.5 at the excitation wavelength. The concentration of 2 was 100 mM in all measurements.
1H NMR experiments
1H NMR spectra (32 scans) were recorded on a 200 MHz Bruker Avance DPX spectrometer. Chemical shifts (δ) are reported in ppm relative to tetramethylsilane (TMS) using the residual undeuterated solvent as an internal reference (acetonitrile-d3, δH = 1.94 ppm).
References
Schmid G (1994) Clusters and colloids: from theory to applications. Wiley, New Jersey
Gates BC (1995) Chem Rev 95:511
Dhas NA, Raj CP, Gedanken A (1998) Chem Mater 10:1446
Minchin RF, Martin DJ (2010) Endocrinology 151:474
Spivey J, Tao F (2014) Metal nanoparticles for catalysis: advances and applications. The Royal Society of Chemistry, p 1
Cushing BL, Kolesnichenko VL, O’Connor CJ (2004) Chem Rev 104:3893
Dahl JA, Maddux BLS, Hutchison JE (2007) Chem Rev 107:2228
Roucoux A, Schulz J, Patin H (2002) Chem Rev 102:3757
Dhas NA, Raj CP, Gedanken A (2006) Chem Mater 9:1446
Mittu R (2016) Int Adv Res J Sci Eng Technol 3:37
Sakamoto M, Fujistuka M, Majima T (2009) J Photochem Photobiol C Photochem Rev 10:33
Yang Y, Liu L, Yin H, Xu D, Liu G, Song X, Liu J (2013) J Phys Chem C 117:11858
Scaiano JC, Aliaga C, Maguire S, Wang D (2006) J Phys Chem B 110:12856
Wee T-L, Sherman BD, Gust D, Moore AL, Moore TA, Liu Y, Scaiano JC (2011) J Am Chem Soc 133:16742
Itakura T, Torigoe K, Esumi K (1995) Langmuir 11:4129
Mcgilvray KL, Decan MR, Wang D, Scaiano JC (2006) J Am Chem Soc 128:15980
Marin ML, Mcgilvray KL, Scaiano JC (2008) J Am Chem Soc 130:16572
Maretti L, Billone PS, Liu Y, Scaiano JC (2009) J Am Chem Soc 131:13972
Miranda OR, Ahmadi TS (2005) J Phys Chem B 109:15724
Yonezawa Y, Sato T, Kuroda S, Kuge K (1991) J Chem Soc, Faraday Trans 87:1905
Kometani N, Doi H, Asami K, Yonezawa Y (2002) Phys Chem Chem Phys 4:5142
Filipescu N, Minn FL (1968) J Am Chem Soc 90:1544
Devadoss C, Fessenden RW (1991) J Phys Chem 95:7253
Evano G, Blanchard N, Toumi M (2008) Chem Rev 108:3054
Gawande MB, Goswami A, Felpin FX, Asefa T, Huang X, Silva R, Zou X, Zboril R, Varma RS (2016) Chem Rev 116:3722
Lignier P, Bellabarba R, Tooze RP (2012) Chem Soc Rev 41:1708
Ingle AP, Duran N, Rai M (2014) Appl Microbiol Biotechnol 98:1001
Bunbury L (1972) Can J Chem 10:2499
Park JW, Kim EK, Park KK (2002) Bull Korean Chem Soc 23:1229
Henglein A (1998) Chem Mater 10:444
Kapoor S, Mukherjee T (2003) Chem Phys Lett 370:83
Kapoor S, Palit DK, Mukherjee T (2002) Chem Phys Lett 355:383
Pacioni NL, Pardoe A, McGilvray KL, Chrétien MN, Scaiano JC (2010) Photochem Photobiol Sci 9:766
Hambrock J, Becker R, Birkner A, Wei J, Fischer RA (2002) Chem Commun 1:68
Chan GH, Zhao J, Hicks EM, Schatz GC, Van Duyne RP (2007) Nano Lett 7:1947
Pootawang P, Saito N, Lee SY (2013) Nanotechnology 24:055604
Scaiano JC (1981) J Phys Chem 85:2851
Soares M, Wasle S, Weil KG, Doblhofer K (2002) J Electroanal Chem 532:353
Nagy Z, Blaudeau JP, Hung NC, Curtiss LA, Zurawski DJ (1995) J Electrochem Soc 142:10
Doblhofer BK, Wasle S, Soares DM, Weil KG, Weinberg G, Ertl G (2003) Z Phys Chem 217:479
Alberti A, Seconi C, Pedulli GF, Degl’Innocenti A (1983) J Organomet Chem 253:291
Dehl R, Fraenkel GK (1963) J Chem Phys 39:1793
Gersdorf J, Mattay J, Goerner H (1987) J Am Chem Soc 109:1203
Porter G, Wilkinson F (1961) TransFaraday Soc 57:1686
Hoshino M, Arai S, Imamura S (1976) J Phys Chem 80:2724
Hu J, Wang J, Nguyen TH, Zheng N (2013) Beilstein J Org Chem 9:1977
Inbar S, Linschitz H, Cohen SG (1981) J Am Chem Soc 103:1048
Kim-Thuan N, Scaiano J (1984) Int J Chem Kinet 16:371
Kausche T, Säuberlich J, Trobitzsch E, Beckert D, Dinse KP (1996) Chem Phys 208:375
Bhattacharyya K, Das PK (1986) J Phys Chem 90:3987
Mukai M, Yamauchi S, Hirota N (1992) T J Phys Chem 6:3305
Okutsu T, Ooyama M, Hiratsuka H (2000) J Phys Chem A 104:288
Hayon E, Ibata T, Lichtin NN, Simica M (1972) J Phys Chem 76:2072
Mukai M, Yamauchi S, Hirota N (1989) J Phys Chem 93:4411
Scaiano JC, Billone P, Gonzalez CM, Maretti L, Marin ML, McGilvray L, Yuan N (2009) Pure Appl Chem 81:635
Mäsing F, Mardyukov A, Doerenkamp C, Eckert H, Malkus U, Nüsse H, Klingauf J, Studer A (2015) Angew Chem Int Ed 54:12612
Acknowledgements
Open access funding provided by Graz University of Technology. We thank NAWI Graz and COST (action CM1201) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schmallegger, M., Gescheidt, G. Benzil/triethylamine: a photo-reducing system for Cu2+. Monatsh Chem 149, 499–504 (2018). https://doi.org/10.1007/s00706-017-2085-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-2085-7