Abstract
Environmentally friendly synthesis of 1,3,2-benzothiazaphosphole-3(2H)-carbothioamide and -diazaphosphole-3(2H)-dicarbothioamide 2-oxides or the carboxamide 2-oxide analogs was reported. The targets were accomplished via a two step process: (1) preparation of the substrates 2-phenyl-2,3-dihydro-1H-1,3,2-benzothiazaphosphole 2-oxide and -1,3,2-benzodiazaphosphole 2-oxide; (2) exploiting these two phospholes in reactions with various saturated and unsaturated isothiocyanates and some isocyanate analogs in dry DMF/pyridine under microwave irradiation thereby isolating the target compounds in 85–95 % yields. Based upon computer-assisted molecular model (CAMM), new compounds were screened for their antidiabetic and antioxidant properties and many of them exhibited moderate to high in vivo and in vitro diabetic and/or antioxidant potencies.
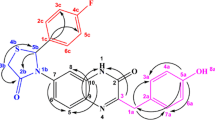
Graphical abstract

Similar content being viewed by others
Introduction
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism. A worldwide survey has reported that diabetes mellitus affects nearly 10 % of the population [1]. The disease results from defects in insulin secretion, insulin action, or both [2]. The epidemic diabetes is accelerating in the developing world, with an increasing proportion of affected people in younger age groups. Recent reports described type-2 diabetes being diagnosed in children and adolescents [3–5].
There are many classes of antidiabetic agents available and these drugs have different mechanisms of action and variable efficacy. However, many patients undergoing diabetic treatment suffer from associated serious side effects [6], emphasizing that the need for new antidiabetic agents with improved efficacy and reduced side effects is still a challenge.
Organophosphorus heterocycles have become notably recognized for their diverse applications in pharmaceutical systems [7, 8]. On the other hand, heterocyclic systems containing phosphorus atom have considerable attention due to the large variety of interesting pharmacological properties [8–10]. Organophosphorus compounds (OPCs) have been known to serve as both hyperglycemic and hypoglycemic agents in different concentrations. As such an increase in blood glucose and decrease in glycogen in various constituents of the brain of rats were observed after treatment with malathion [11]. In another report, glycogen levels had decreased in the liver of rats when treated with dichlorofos [12]. Furthermore, diisopropyl phosphorofluoridate has the ability to reduce the glucose level in rats [13]. In the same context, phosphites and phosphonates exhibited potent antioxidant effects, and are acting as both primary and secondary antioxidants [14, 15].
In sequel, the work herein was designed to synthesize new 1,3,2-thiaza/dicarbothioamide 2-oxides or the carboxamide 2-oxide analogs and to bioassay their antidiabetic and antioxidant activities. The optimized pharmacological evaluation of new OPCs based on the prospective predicted potency was carried out using the computer-assisted molecular modeling (CAMM) [16, 17]. This study is a part of our continuous interest in synthesis of a wide range of heterocycle-phosphor ester systems for biological screening programs [18–23].
Results and discussion
Chemistry
In the last few years, the application of microwave irradiation in synthetic organic chemistry has become more and more interesting. Microwave-assisted synthesis offers a versatile and a facile pathway for a large variety of syntheses. Thus, a large number of organic reactions were preferably carried out under microwave irradiation due to higher yields, short reaction times and friendly conditions of this process [24].
Motivated by the aforementioned findings, the synthesis of substituted 1,3,2-thiazaphosphole 2-oxides 5a–5e was accomplished through two step process (Scheme 1). The required 1,3,2-thiazaphosphole 2-oxide 3 was prepared by a slight modification to the procedures reported in the literature [25, 26]. While in the first method [25] the P(V) phosphonyl dichloride was used in a general procedure to prepare about thirty phosphole 2-oxide derivatives, in the second procedure [26] phosphole analogs, e.g. 1,3-dihydro-1,3,2-benzodiazaphosphole 2-oxide were obtained from the reaction of parent diamine and P(III) diphenyl phenylphosphonite. However, in our experiment we treated 2-aminobenzenethiol (1) with dichlorophenylphosphine (2) in tetrahydrofuran (THF) at room temperature (r.t.) to afford 3 in 61 % yield (Scheme 1). Compound 3 is compatible with the previously reported data [25] (see Figs. 1, 2). The oxidation of the expected phosphole to its oxide form in the previous reaction is attributed to the presence of fortuitous water [26] and the affinity of P(III) to oxygen to establish the more stable P(V) form [27]. However, executing the previous reaction in the presence of 1 cm3 of H2O2, the yield of 3 is enhanced to 72 %. On the other hand, when the reaction was carried out under oxygen free condition, only the phosphole (and not the phosphole oxide) is obtained (see experimental section).

In the second stage, compound 3 was subjected to reactions with various isothiocyanates 4a–4d and the isocyanate analog 4e in dimethylformamide (DMF) containing a catalytic amount of pyridine to obtain the target compounds 5a–5e in excellent yields (>90 %). The second step of the methodology was completed under microwave (MW) irradiation within 4–6 min. Compounds 5a–5e were formed via a direct conjugate addition of the phosphole 2-oxide 3 with the hard electrophiles, isothio- and isocyanates 4a–4d/4e [28–30].
1,3,2-Benzothiazaphosphole-3(2H)-carbothioamide 2-oxides 5a–5d and carboxamide 5e exhibited IR absorption bands for P=O, C=S (or C=O, 5e), and NH in the regions 1196-1180, 1222-1149 (5e: 1728,) and 3423-3348 cm−1, respectively. The NH proton gave a singlet at δ = 10.55 ppm while the methyl protons (NMe) resonated at 3.35 ppm as a singlet in the 1H NMR spectrum of 5a. In its 13C NMR spectrum, the C(S) and P–C(1′) appeared as two doublets at δ = 186.4 ppm (2 J PC = 11.3 Hz) and 130.4 ppm (d, 1 J PC = 122 Hz), while the methyl-carbon showed a doublet at 30.9 ppm (4 J PC = 4.2 Hz, Me–N) ppm. The remaining carbon resonances were observed in the expected region. 31P NMR signals of 5a–5e were observed in the region 51.6–56.8 ppm [31].
We also conducted the same reaction with 1,3,2-benzodiazaphosphole 2-oxide (7), which was obtained as previously mentioned to 3, under microwave irradiation to give 1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxides 8a–8d and carboxamide 8e in >85 % yield. In this case, the reaction between 7 and the isothio-4a–4d/isocyanates 4e was completed when only two moles of 4 were used in the reaction (Scheme 2).

The structural elucidation of 8 was straightforward, since the EI-MS data of 8a–8e showed correct molecular ions. The 1H NMR spectrum of 8a showed broad characteristic signal at 12.56 ppm for the two NH protons and two singlets at 3.24, 3.45 ppm assigned to the two methyl protons. Its 13C NMR spectrum had among others, a doublet (1 J PC = 155 Hz) centered at 130.8 ppm assigned to P–C(1′) moiety and only one doublet (2 J PC = 8.7 Hz) at 188.2 ppm due to the two C(S) moieties due to the symmetry of the molecule.
In a systemic study equivalent amounts of 3 and allylisothiocyanate (9) were reacted under the same reaction conditions to give a product for which structure 10 was assigned for the following reasons. The 1H NMR spectrum of 10 showed the methyl protons as a doublet (J HH = 8.1 Hz) at 2.04 ppm and the NH proton as a broad signal at 10.75 ppm. On the other hand, the methylene protons (2H) present in the 1H NMR spectrum of 9 as a doublet (J HH = 8.1 Hz) at 4.27 ppm were absent in the spectrum of 10. Instead, each of the exocyclic methine protons (2H) in 10 appeared differently. That of proton (a) appeared as a doublet of quartet (dq, J HH = 8.1, 4.2 Hz) at 4.18 ppm while the other proton (b) showed a doublet of doublet (J HH = 12.0 Hz, E-form) at 6.33 ppm. These data were also confirmed in the 13C NMR spectrum. The presence of the AB system and the lack of signals due to the methylene (–CH2) and the methylidene (=CH2) groups in the 1H and 13C NMR spectral data confirmed the assigned structure 10, 2-phenyl-N-[(1E)-prop-1-en-1-yl]-1,3,2-benzothiazaphosphole-3(2H)-carbothioamide 2-oxide, and ruled out the other alternative structure like 11 (Scheme 3).

In a similar fashion, 2-phenyl-N,N′-bis[(1E)-prop-1-en-1-yl]-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxide (12, 86 % yield) was obtained from the reaction of 7 with two moles of allylisothiocyanate (9) using the same reaction conditions Fig. 3.
It is noteworthy that in a blank experiment, benzothiaza-3 and benzodiazaphosphole 2-oxide 7 were recovered practically unchanged when a solution of each in DMF/pyridine was subjected to MW irradiation for 7 min. Furthermore, in a parallel experiment, the phosphole 2-oxides 3 and 7 were allowed to react with methyl isothiocyanate 4a, as a representative example, in DMF solution containing pyridine under thermal condition for ≈8 h (TLC). After the usual working up, products 5a and 8a were obtained in 56 and 46 % yields.
Structures of all synthesized compounds were confirmed by 1H, 13C, 31P NMR, and MS data as well as IR spectra.
Biological activity spectra prediction
Prospective biological activity spectra for the molecular structures 5a–5e, 8a–8e, 10, 12, and the substrates 3 and 7 were predicted in the early stage of the investigation. The computer assisted molecular modeling (CAMM) program (PASS, 2012.1 version, IBMC, Moscow, Russia) was adopted for designing the structures in silico [16, 17]. The spectrum for a substance is a list of the biological activity types for which the probability to be revealed (Pa) and the probability not to be revealed (Pi) are calculated. Pa and Pi values are independent and their values vary from 0 to 1 (Supplementary Material, appendix 1). By default, in PASS, Pa = Pi value is chosen as a threshold, therefore all compounds with Pa > Pi are suggested to be active.
The study indicated that the main expected biological activity of the designed compounds were the antidiabetic and antioxidant activities, which they had in common.
Antidiabetic activity
Type-2 diabetes mellitus (non-insulin dependent diabetes mellitus, NIDDM) is a much more prevalent form of regular diabetes and is responsible for 90 % of the disease prevalence [32–34]. Therefore, experimental animal model representing type-2 diabetes, streptozotocin (STZ) diabetic rats, is used to assess the antidiabetic efficacy of the tested compounds. This animal model develops most of the biochemical and pathological symptoms associated with type-2 diabetes in humans [35].
Accordingly, the antidiabetogenic effect of synthesized thiazaphospholes 5a–5e, 10, and diazaphospholes 8a–8e and 12 has been investigated at a dose of 20 mg/kg. The tested compounds were evaluated in DMSO solution on STZ-induced diabetic rats in time duration dependent fashion. After 7 and 21 days of administration with DMSO solution of the tested compounds, the fasting blood glucose (FBG) level was collected from the animal of all groups, and was measured by single touch glucometer [36]. The results presented in Table 1, while the percentage potency of the tested phospholes vs. FBG of diabetic rats was displayed in Fig. 4. Glibenclamide drug (Z) was used as a reference standard.
A significant diminution of FBG level was observed in respect to the diabetic rats, but there was no significant alteration of FBG level to the control. FBG level of all animals before treatment was within the normal range whereas FBG level was significantly elevated after 24 h of STZ injection with respect to the control level.
Data in Table 1 shows that 8a, 8b, 12 exhibited greater extent of hypoglycemic activity when compared with the remaining compounds or the reference drug. However, most of the tested compounds displayed moderated efficacy: 5a > 10 > 8c > 5b > 5c > 8d > 5d > 8e > 5e. The highest activity of 8a might be due to the presence of two of N–C(S)–N moieties along with a small alkyl group such as methyl fragment substituent at the thiocyanate motif. On the other hand, the presence of the phenyl fragment as a substituent reduced the potency dramatically.
Toxicity of the promised product
For determination of lethal dose (LD50) of 8a, single gradual increasing doses were administered to various groups of normal albino mice. The number of dead animals in each group was determined after 48 h of compound administration and LD50 was calculated. LD50 of 8a was found to be 1578 mg/kg b.w. for mice. Using the conversion equation [37], LD50 for rats was found to be 1096 mg/kg b.w. Based on this toxicity study, the orally therapeutic dose for subsequent in vivo study was chosen to be 20 mg/kg b.w. (~1/50 of LD50), which is very far below LD50.
Antioxidant evaluation
The studied compounds were screened for their antioxidant activity using ABTS method which was reported by Lissi et al. [38]. The assay employed here is one of several assays that depends on measuring the consumption of stable free radicals, i.e., evaluates the free radical scavenging activity of the investigated compounds 5a–5e, 8a–8e, 10, and 12. The methodology assumes that the consumption of the stable free radicals (X− ) will be determined by reactions as follows:
The rate and/or the extent of the process measured in terms of the decrease in X− concentration would be related to the ability of the added compounds to trap free radicals. The decrease in color intensity of the free radical solution due to the scavenging of the free radicals by the antioxidant material is measured calorimetrically at a specific wavelength. The assay employed the radical cation derived from 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid, ABTS) as stable free radical to assess antioxidant potential of the investigated compounds.
Some of the corresponding phosphole 2-oxide derivatives displayed antioxidant activity compared to ascorbic acid as shown in Table 2. Compounds 5b, 8b, 8c, 10, and 12 displayed high antioxidant effects, while compounds 5a, 5c, and 8a showed moderate antioxidant activity. On the other hand, compounds 5d, 5e, 8d, and 8e showed only a weak potency. Compounds 10 and 12 exhibited the highest potency compared with other compounds.
Bleomycin-dependent DNA damage assay
The bleomycin is a family of glycopeptide antibiotics that is used routinely as antitumor agent. The bleomycin assay was adopted for assessing the pro-oxidant effects of food antioxidant. The antitumor/antibiotic bleomycin binds iron ions and DNA. The bleomycin-iron complex degrades DNA that, on heating with thiobarbituric acid (TBA), yields a pink chromogen. Upon the addition of a suitable reducing agent, the antioxidant competes with DNA and diminishes the chromogen formation [39].
The protective activity against DNA damage induced by bleomycin-iron complex was examined to show the mechanism of action of the potent compounds 5b, 8b, 8c, 10, and 12. Data in Table 3 shows that compounds 10 and 12 exhibited highest protection against DNA damage induced by the bleomycin-ion complex, which indicated diminishing chromogen formation between damaged DNA and phosphorus molecules.
By comparing the results obtained for the antioxidant properties of the compounds reported in this study with their structures, the flow of the structure activity relationships (SARs) was displayed in Fig. 5. Finally, the phosphole 2-oxides 10 and 12 are more potent than ascorbic acid, which may be attributable to the presence of exocyclic unsaturated moiety other than the phosphole motif.
Conclusion
In conclusion, the present investigation afforded an efficient, and environmentally benign methodology toward the synthesis of 1,3,2-benzothiazaphosphole-3(2H)-carbothioamide-, -diazaphosphole-3(2H)-dicarbothioamide 2-oxides and some of their carboxamide analogs. The process involved the reactions of 1,3,2-benzothiaza- and 1,3,2-benzodiazaphosphole 2-oxides with a variety of saturated and unsaturated isothio and isocyanates under microwave-assisted condition. The notable advantages of this procedure are: (1) general applicability of nucleophiles with hard electrophilic isothiocyanates, (2) almost quantitative yields of the products (85–95 %), (3) very short reaction time, (4) operational simplicity, (5) less solvent-consuming and catalyst-free conditions, (6) the present methodology showed that the phosphole ring is tenable under the used reaction conditions. Furthermore, type-2 diabetes mellitus (non-insulin dependent diabetes mellitus, NIDDM) and antioxidant evaluation of the products showed that the diazaphosphole-3(2H)-carbothioamide 2-oxides displayed higher potency compared with the thiazaphosphole-counterparts. This observation indicates that increasing nitrogen atoms in the phosphole ring enhanced the pharmacological activity.
Experimental
Melting points were determined with open capillary tube on an Electrothermal (variable heater) melting point apparatus and were corrected. IR spectra were recorded on a JASCO FT-IR 6100 using KBr disk (JASCO, Japan). NMR spectra were measured with a JEOL E.C.A-500 MHz (13C: 125.7 MHz, 1H: 500 MHz, 31P: 202.4 MHz) spectrometer (JEOL, Japan). 31P NMR spectra were recorded with H3PO4 (85 %) as external reference. 1H and 13C NMR spectra were recorded with tetramethylsilane as internal standard in DMSO-d 6 . Chemical shifts (δ) are given in ppm. The mass spectra were performed at 70 eV on an MS-50 Kratos (A.E.I.) spectrometer provided with a data system [spectrometer (Kratos, UK)]. Elemental analyses were carried out at the Microanalysis Laboratory, Cairo University, Cairo, Egypt, using elementary Analysen-systeme GmbH-vario EL III Element Analyzer, Germany. The appropriate precautions in handling moisture-sensitive compounds were observed. The purity of all new samples was verified by microchemical analysis (C/H/N/S) and spectroscopy. Solvents were dried by standard techniques, TLC: Merck 0.2 mm silica gel 60 F254 analytic aluminum plates. The microwave oven used is a Milestone Italy (model: StartSynth, Reactor: Pack2B Basic Single Vessel Kit).
Preparation of phosphole 2-oxides 3 and 7
A mixture of 3.75 g 2-aminobenzenethiol (1, 30 mmol) and 3.9 g dichlorophenylphosphine (0.01 mmol) in 30 cm3 THF (98 %) was stirred at r.t. for 30 min. After removal of the solvent, the residue was collected and crystallized from EtOH to afford 2-phenyl-3H-l,3,2-benzothiazaphosphole 2-oxide (3) as white substance (4.6 g, 61 %). M.p.: 190 °C (Ref. [40] 190 °C).
Similarly, the reaction between 3.24 g benzene-1,2-diamine (6, 30 mmol) and 3.9 g dichlorophenyl phosphine (2, 30 mmol) in 30 cm3 THF, under the conditions described above, afforded 1,3-dihydro-2-phenyl-1,3,2-benzodiazaphosphole 2-oxide (7) as orange substance (4.1 g, 60 %). M.p.: 275 °C (Ref. [26] 275 °C).
When the above reactions (preparation of 3 or 7) were carried out in THF solution containing 1 cm3 H2O2 using the same amounts at r.t. for ≈10–15 min (TLC), the substrates 3 and 7 were obtained after the usual working up., as sole reaction products. Yields: 3 (72 %), 7 (75 %).
On the other hand, when the reaction of quantitative amounts of 1 and 2 in THF solution was carried out under oxygen free condition, only the corresponding 1,3,2-benzothiazaphosphole (and not the phosphole oxide) is obtained: m.p.: 148 °C (Ref. [26] 148 °C).
Preparation of carbothioamide 2-oxides 5a – 5d, 10 and carboxamide 5e
2-Phenyl-2,3-dihydro-1,3,2-benzothiazaphosphole 2-oxide (3, 0.4 g, 1.6 mmol) in 5 cm3 dry DMF was placed, with a magnetic stirring bar, into a microwave quartz vessel. Isothiocyanates: methyl, ethyl, cyclohexane, phenyl isothiocyanate (4a–4d), allylisothiocyanate (9), or phenylisocyanate (4e) (1.6 mmol) was added, followed by addition of few drops of pyridine. The reaction mixture was heated in the microwave reactor at 140 °C for the suitable hold time (4–6 min). After the completion of the reaction (TLC), the product mixture was allowed to cool down, poured into ice-water, and acidified with conc. HCl. The resulting residue was crystallized from suitable solvent to afford the corresponding products 5a–5d, 10, and 5e.
N-Methyl-2-phenyl-1,3,2-benzothiazaphosphole-3(2H)-carbothioamide 2-oxide (5a, C14H13N2OPS2)
Recrystallization from EtOH afforded a white substance. Yield 0.47 g (91 %); m.p.: 177 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 3.35 (s, 3H, Me–N), 6.96–7.63 (m, 9H, H-Ph, H-Ar), 10.55 (br, 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 186.4 (d, 2 J PC = 11.3 Hz, C=S), 140.3 (d, 2 J PC = 14.5 Hz, C(9)), 131.7 (d, 2 J PC = 12.8 Hz, C(8)), 131.6 (d, 4 J PC = 4.1 Hz, C(4′)), 130.4 (d, 1 J PC = 122 Hz, P–C(1′)), 129.8 (d, 3 J PC = 10.4 Hz, C(7)), 129.5 (d, 3 J PC = 10.3 Hz, C(3′,5′)), 129.4, 128.6 (2d, 2 J PC = 12.4 Hz, C(2′,6′)), 125.7 (d, 4 J PC = 3.8 Hz C(5)), 123.8 (d, 4 J PC = 3.8 Hz, C(6)), 117.7 (d, 3 J PC = 8.8 Hz, C(4)), 30.9 (d, 4 J PC = 4.2 Hz, Me–N) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 52.4 ppm; IR (KBr): \(\bar{\nu }\) = 3423 (NH), 1195 (P=O), 1154 (C=S) cm−1; MS (EI, 70 eV): m/z (%) = 320 (M+, 62), 246 (100).
N-Ethyl-2-phenyl-1,3,2-benzothiazaphosphole-3(2H)-carbothioamide 2-oxide (5b, C15H15N2OPS2)
Recrystallization from MeCN afforded a white substance. Yield 0.5 g (93 %); m.p.: 163 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 1.52 (t, 3H, J HH = 6.6 Hz, MeC–N), 3.60 (q, 2H, J HH = 6.6 Hz, H2C–N), 7.33–7.64 (m, 9H, H-Ph, H-Ar), 9.89 (br, 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 185.8 (d, 2 J PC = 12.1 Hz, C=S), 140.2 (d, 2 J PC = 12.5 Hz, C(9)), 131.7 (d, 2 J PC = 14.8 Hz, C(8)), 131.4 (d, 4 J PC = 4.4 Hz, C(4′)), 130.4 (d, 1 J PC = 143 Hz, P–C(1′)), 129.8 (d, 3 J PC = 10.4 Hz, C(7)), 129.8 (d, 3 J PC = 8.4 Hz, C(3′,5′)), 129.4, 128.3 (d, 2 J PC = 12.4 Hz, C(2′,6′)), 125.7 (d, 4 J PC = 3.8 Hz C(5)), 123.7 (d, 4 J PC = 3.8 Hz, C(6)), 117.7 (d, 3 J PC = 8.8 Hz, C(4)), 39.7 (d, 4 J PC = 4.3 Hz, CH2–N), 17.3 (s, Me) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 51.6 ppm; IR (KBr): \(\bar{\nu }\) = 3426 (NH), 1187 (P=O), 1212 (C=S) cm−1; MS (EI, 70 eV): m/z (%) = 334 (M+, 58), 246 (100).
N-Cyclohexyl-2-phenyl-1,3,2-benzothiazaphosphole-3(2H)-carbothioamide 2-oxide (5c, C19H21N2OPS2)
Recrystallization from pentane afforded a straw yellow substance. Yield 0.59 g (95 %); m.p.: 63 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 0.99–1.96 (m, 10H, H2C-chex), 3.38 (m, 1H, H-chex), 7.24–7.85 (m, 9H, H-Ph, H-Ar), 10.70 (br, 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 188.6 (d, 2 J PC = 12.8 Hz, C=S), 139.6 (d, 2 J PC = 12.6 Hz, C(9)), 131.3 (d, 2 J PC = 14.8 Hz, C(8)), 130.5 (d, 4 J PC = 4.6 Hz, C(4′)), 129.3 (d, 4 J PC = 3.8 Hz, C(6)), 129.7 (d, 1 J PC = 148 Hz, P–C(1′)), 129.4 (d, 3 J PC = 10.4 Hz, C(7)), 128.3 (d, 3 J PC = 8.3 Hz, C(3′,5′)), 127.9 (d, 2 J PC = 12.4 Hz, C(2′,6′)), 125.5 (d, 4 J PC = 4.0 Hz, C(5)), 123.7 (d, 4 J PC = 3.8 Hz, C(6)), 117.7 (d, 3 J PC = 8.8 Hz, C(4)), 54.2 (d, 4 J PC = 2.8 Hz, CH-chex), 39.7, 25.4, 24.2 (3 s, CH2-chex) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 56.2 ppm; IR (KBr): \(\bar{\nu }\) = 3348 (NH), 1194 (P=O), 1149 (C=S) cm−1; MS (EI, 70 eV): m/z (%) = 388 (M+, 60), 246 (100).
N,2-Diphenyl-1,3,2-benzothiazaphosphole-3(2H)-carbothioamide 2-oxide (5d, C19H15N2OPS2)
Recrystallization from MeCN afforded a straw yellow substance. Yield 0.55 g (90 %); m.p.: 165 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 7.11–7.77 (m, 14H, H-Ph, H-Ar), 10.50 (br, 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 184.8 (d, 2 J PC = 11.4 Hz, C = S), 143.2 (d, 2 J PC = 14.4 Hz, C(9)), 132.2 (d, 2 J PC = 14.8 Hz, C(8)), 131.2 (d, 4 J PC = 4.9 Hz, C(4′)), 130.4 (d, 1 J PC = 148 Hz, P–C(1′)), 130.0 (d, 3 J PC = 10.4 Hz, C(7)), 128.3 (d, 3 J PC = 8.5 Hz, C(3′,5′)), 129.4, 128.6 (d, 2 J PC = 12.2 Hz, C(2′,6′)), 125.5 (d, 4 J PC = 4.0 Hz, C(5)), 123.8 (d, 4 J PC = 3.8 Hz, C(6)), 117.3 (d, 3 J PC = 8.8 Hz, C(4)), [138.4 (d, 4 J PC = 3.8 Hz, C(1a)), 127.1, 125.5, 122.0 (C(2a–6a)] ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 56.8 ppm; IR (KBr): \(\bar{\nu }\) = 3423 (NH), 1190 (P=O), 1192 (C=S) cm−1; MS (EI, 70 eV): m/z (%) = 382 (M+, 48), 246 (100).
N,2-Diphenyl-1,3,2-benzothiazaphosphole-3(2H)-carboxamide 2-oxide (5e, C19H15N2 O2PS)
Recrystallization from cyclohexane afforded a pale yellow 0.54 g (92 %); m.p.: 75 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 7.29–7.48 (m, 14H, H-Ph, H-Ar), 10.24 (br, 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 145.2 (d, 2 J P–C = 8.8 Hz, C=O), 138.3 (d, 2 J PC = 13.4 Hz, C(9)), 135.2 (d, 2 J PC = 14.8 Hz, C(8)), 133.5 (d, 1 J PC = 128 Hz, P-C(1′)), 131.2 (d, 4 J PC = 4.6 Hz, C(4′)), 130.0 (d, 3 J PC = 10.4 Hz, C(7)), 129.8 (d, 3 J PC = 8.4 Hz, C(3′,5′)), 128.9 (d, 2 J PC = 12.5 Hz, C(2′,6′)), 125.5 (d, 4 J PC = 4.0 Hz, C(5)), 123.2 (d, 4 J PC = 3.8 Hz, C(6)), 117.3 (d, 3 J PC = 8.8 Hz, C(4)), [138.1 (d, 4 J PC = 3.4 Hz, C(1a)), 127.3, 124.7, 121.4 (C(2a-6a)] ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 54.4 ppm; IR (KBr): \(\bar{\nu }\) = 3407 (NH), 1728 (C=O), 1186 (P=O) cm−1; MS (EI, 70 eV): m/z (%) = 366 (M+, 65), 246 (100).
2-Phenyl-N-[(1E)-prop-1-en-1-yl]-1,3,2-benzothiazaphosphole-3(2H)-carbothioamide 2-oxide (10, C16H15N2OPS2)
Recrystallization from CHCl3 afforded a pale yellow substance. Yield 0.52 g (88 %); m.p.: 195 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.04 (d, 3H, J HH = 8.1 Hz, Me), 4.18 (dq, 1H, J HH = 8.1, 4.2 Hz, Ha), 6.33 (dd, 1H, J HH = 12.0 Hz, Hb), 7.06–7.63 (m, 9H, H-Ph, H-Ar), 10.75 (br, 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 179.4 (d, 2 J PC = 11.6 Hz, C=S), 140.6 (d, 2 J PC = 12.2 Hz, C(9)), 137.4 (d, 4 J PC = 3.8 Hz, N–C=C, exocyclic), 133.6 (d, 2 J PC = 12.8 Hz, C(8)), 131.7 (d, 4 J PC = 4.6 Hz, C(4′)), 129.4 (d, 3 J PC = 10.4 Hz, C(7)), 128.3 (d, 3 J PC = 8.3 Hz, C(3′,5′)), 127.9 (d, 1 J PC = 148 Hz, P–C(1′)), 129.4, 126.8 (d, 2 J PC = 12.6 Hz, C(2′,6′)), 125.5 (d, 4 J PC = 4.0 Hz, C(5)), 123.5 (d, 4 J PC = 3.8 Hz, C(6)), 120.4 (d, 3 J PC = 10.8 Hz, C(4)), 110.7 (s, CHMe), 14.5 (s, Me) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 51.2 ppm; IR (KBr): \(\bar{\nu }\) = 3367 (NH), 1220 (C=S), 1180 (P=O) cm−1; MS (EI, 70 eV): m/z (%) = 346 (M+, 62), 246 (100).
Preparation of carbothioamide 2-oxides 8a–8d, 12 and carboxamide 8e
Benzodiazaphosphole 2-oxide 7 (0.4 g, 1.75 mmol) and two molar equivalent (3.5 mmol) of the isothiocyanates 4a–4d, 9 or isocyanate 4e (3.5 mmol) in 7 cm3 dry DMF were caused to react under the MW irradiation (5–7 min) under the same previous reaction conditions. After the completion of the reaction (TLC), the product mixture was allowed to cool down, poured into ice-water, and acidified with conc. HCl. The resulting residue was crystallized from suitable solvent to give the corresponding products 8a–8d, 12, and 8e.
N,N′-Dimethyl-2-phenyl-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxide (8a, C16H17N4OPS2)
Recrystallization from CHCl3 afforded a yellow substance. Yield 0.57 g (88 %); m.p.: 289 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 3.24, 3.45 (2s, 2 × 3H, 2 Me–N), 7.22–7.88 (m, 9H, H-Ph, H-Ar), 12.56 (br, 2 × 1H, 2 HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 188.2 (d, 2 J PC = 11.8 Hz, 2 C = S), 133.3 (d, 2 J PC = 14.5 Hz, C(8,9)), 131.8 (d, 2 J PC = 12.4 Hz, C(2′,6′)), 131.5 (d, 4 J PC = 4.9 Hz, C(4′)), 130.8 (d, 1 J PC = 155 Hz, P–C(1′)), 129.8 (d, 3 J PC = 10.4 Hz, C(3′,5′)), 124.4 (d, 4 J PC = 3.5 Hz C(5,6)), 119.7 (d, 3 J PC = 8.8 Hz, C(4,7)), 32.9 (d, 4 J PC = 4.2 Hz, 2 Me–N) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 54.4 ppm; IR (KBr): \(\bar{\nu }\) = 3374–3350 (2 NH), 1182 (P=O), 1154, 1201 (2 C=S) cm−1; MS (EI, 70 eV): m/z (%) = 376 (M+, 43), 228 (100).
N,N′-Diethyl-2-phenyl-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxide (8b, C18H21N4OPS2)
Recrystallization from CHCl3 afforded a yellow substance. Yield 0.64 g (92 %); m.p.: 234 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 1.29, 1.41 (2t, 2 × 3H, J = 7.8 Hz, 2 MeC–N), 3.56–3.69 (m, 2 × 2H, 2 H2C-N), 7.21-7.72 (m, 9H, H-Ph, H-Ar), 12.21 (br, 2 × 1H, 2 HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 188.8 (d, 2 J PC = 10.9 Hz, 2 C=S), 134.2 (d, 2 J PC = 12.5 Hz, C(8,9)), 131.3 (d, 2 J PC = 12.3 Hz, C(2′,6′)), 131.4 (d, 4 J PC = 4.8 Hz, C(4′)), 130.4 (d, 1 J PC = 143 Hz, P–C(1′)), 129.5 (2d, 3 J PC = 8.5 Hz, C(3′,5′)), 124.7 (d, 4 J PC = 3.8 Hz C(5,6)), 118.4 (d, 3 J PC = 8.8 Hz, C(4,7)), 38.7 (d, 4 J PC = 4.4 Hz, 2 CH2–N), 18.3 (s, 2 Me) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 53.7 ppm; IR (KBr): \(\bar{\nu }\) = 3436–3389 (2 NH), 1196 (P=O), 1208, 1228 (2 C=S) cm−1; MS (EI, 70 eV): m/z (%) = 404 (M+, 39), 228 (100).
N,N′-Dicyclohexyl-2-phenyl-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxide (8c, C26H33N4OPS2)
Recrystallization from MeOH afforded a pale yellow substance. Yield 0.76 g (86 %); m.p.: 166 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 1.09–1.66 (m, 2 × 10H, H2C-chex), 3.78–3.81 (m, 2 × 1H, H-chex), 7.25–7.77 (m, 9H, H-Ph, H-Ar), 12.52 (br, 2 × 1H, 2 HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 192.2 (d, 2 J PC = 10.4 Hz, 2 C = S), 133.6 (d, 2 J PC = 11.8 Hz, C(8,9)), 131.6 (d, 3 J PC = 8.6 Hz, C(2′,6′)), 130.8 (d, 4 J PC = 4.6 Hz, C(4′)), 130.3 (d, 1 J PC = 163 Hz, P–C(1′)), 128.8 (d, 3 J PC = 8.2 Hz, C(3′,5′)), 124.5 (d, 4 J PC = 4.0 Hz, C(5,6)), 119.6 (d, 3 J PC = 8.8 Hz, C(4,7)), 57.4 (d, 4 J PC = 2.8 Hz, N–CH–chex), 39.7, 25.4, 24.2 (3 s, CH2–chex) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 52.1 ppm; IR (KBr): \(\bar{\nu }\) = 3413–3945 (2 NH), 1194 (P=O), 1146, 1210 (2 C=S) cm−1; MS (EI, 70 eV): m/z (%) = 512 (M+, 37), 228 (100).
N,N′-Diphenyl-2-phenyl-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxide (8d, C26H21N4OPS2)
Recrystallization from MeOH afforded a yellow substance. Yield 0.79 g (91 %); m.p.: 265 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 7.26–7.90 (m, 19H, m, H-Ph, H-Ar), 12.02 (br, 2 × 1H, 2 HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 188.8 (d, 2 J PC = 11.8 Hz, 2 C=S), 133.4 (d, 2 J PC = 11.8 Hz, C(8,9)), 131.2 (d, 2 J PC = 12.6 Hz, C(2′,6′)), 130.7 (d, 4 J PC = 4.6 Hz, C(4′)), 130.3 (d, 1 J PC = 160.4 Hz, P–C(1′)), 129.8 (d, 3 J PC = 8.2 Hz, C(3′,5′)), 124.1 (d, 4 J PC = 4.0 Hz, C(5,6)), 119.6 (d, 3 J PC = 8.8 Hz, C(4,6)), [137.8 (d, 4 J PC = 3.8 Hz, C(1a)), 125.6, 124.8, 122.3, 120.6 (C(2a–6a)] ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 53.8 ppm; IR (KBr): \(\bar{\nu }\) = 3431 (2 NH), 1190 (P=O), 1213, 1175 (2 C=S) cm−1; MS (EI, 70 eV): m/z (%) = 500 (M+, 25), 228 (100).
N,N′-Diphenyl-2-phenyl-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarboxamide 2-oxide (8e, C26H21N4O3P)
Recrystallization from MeOH afforded a yellow substance. Yield 0.73 g (91 %); m.p.: 157 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 7.20–7.87 (m, 19H, H-Ph, H-Ar), 11.80 (2br, 2 × 1H, HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 150.3 (d, 2 J PC = 10.2 Hz, 2 C=O), 134.6 (d, 1 J PC = 154.4 Hz, P–C(1′), 131.6 (d, 2 J PC = 14.8 Hz, C(8,9)), 130.5 (d, 4 J PC = 3.9 Hz, C(4′)), 127.8 (d, 3 J PC = 8.1 Hz, C(3′,5′)), 127.9 (d, 2 J PC = 12.2 Hz, C(2′,6′)), 125.5 (d, 4 J PC = 4.0 Hz, C(5,6)), 120.6 (d, 3 J PC = 8.8 Hz, C(4,7)), [138.4 (d, 4 J PC = 3.8 Hz, 2 C(1a)), 128.4, 127.6, 127.1, 125.6, 124.4, 122.0, 118.6 (C(2a–6a)] ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 54.1 ppm; IR (KBr): \(\bar{\nu }\) = 3425, 3400 (2 NH), 1738, 1727 (2 C=O), 1194 (P=O) cm−1; MS (EI, 70 eV): m/z (%) = 468 (M+, 65), 228 (100).
2-Phenyl-N,N′-bis[(1E)-prop-1-en-1-yl]-1H-1,3,2-benzodiazaphosphole-1,3(2H)-dicarbothioamide 2-oxide (12, C20H21N4OPS2)
Recrystallization from MeOH afforded a straw yellow substance. Yield 0.63 g (86 %); m.p.: 212 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ = 2.01, 2.23 (2d, 2 × 3H, J HH = 8.4 Hz, 2 MeN), 4.15, 4.26 (2 dq, 2 × 1H, J HH = 8.4, 4.3 Hz, Ha), 6.21, 6.45 (2dd, 2 × 1H, J HH = 12.8, Hb), 7.06–7.63 (m, 9H, H-Ph, H-Ar), 10.90 (br, 2 × 1H, 2 HN) ppm; 13C NMR (125.7 MHz, DMSO-d 6 ): δ = 192.3 (d, 2 J PC = 12.4 Hz, 2 C=S), 137.4 (d, 2 J PC = 14.8 Hz, 2 N–C=C, exocyclic), 136.4 (d, 1 J PC = 158 Hz, P–C(1′)), 134.6 (d, 2 J PC = 14.8 Hz, C(8,9)), 132.8 (d, 2 J PC = 12.8 Hz, C(2′,6′)), 131.7 (d, 4 J PC = 3.9 Hz, C(4′)), 128.3 (d, 3 J PC = 8.1 Hz, C(3′,5′)), 124.5 (d, 4 J PC = 4.0 Hz, C(5,6), C(6)), 120.4 (d, 3 J PC = 10.8 Hz, C(4,7)), 110.7 (s, 2 CHMe), 14.5 (s, 2 Me) ppm; 31P NMR (202.4 MHz, DMSO-d 6 ): δ = 54.4 ppm; IR (KBr): = 3411, 3383 (2 NH), 1205, 1180 (2 C=S), 1190 (P=O) cm−1; MS (EI, 70 eV): m/z (%) = 428 (M+, 62), 228 (100).
Parallel thermal experiment
A stirred mixture of equivalent amounts of benzothiazaphosphole 2-oxide 3 (or benzodiazaphosphole 2-oxide 7) and methyl isothiocyanates 4a in 10 cm3 DMF, was followed by adding few drops of pyridine. The reaction mixture was heated under reflux for 6-8 h (TLC). After removal of the volatile materials under vacuum, the residual substance was crystallized from the proper solvent to give the corresponding products 5a or 8a in 56 and 46 % yields (TLC, m.p., mixed m.ps, and comparative IR spectra).
Blank experiment
A sample of 0.2 g benzothiazaphosphole 2-oxide (3, 0.8 mmol) or 0.2 g benzodiazaphosphole 2-oxide (7, 0.87 mmol) in 5 cm3 dry DMF was placed, with a magnetic stirring bar into the MW-quartz vessel, followed by addition of few drops of pyridine. The reaction mixture was heated in the MW reactor at 140 °C for 7 min hold time. After removal of the volatile materials under vacuum, the residual substance was crystallized from EtOH to give, practically unchanged 3 or 7 (TLC, m.p., mixed m.ps, and comparative IR spectra).
Experimental animals
Experimental albino mice (weighing 20–25 g) were obtained from the Animal House of Ophthalmology Institute, Giza, Egypt. They were kept under observation for 2 weeks in the department animal house to exclude any inter-current infection. They were supplied standard diet and tap water ad libitum, and maintained under suitable living conditions in good aerated cages at natural daily 12 h dark-light cycle and at r.t. 20–25 °C. All international principles and local regulations concerning the care and the use of laboratory animals were considered during the pharmacological screening.
Antidiabetes evaluation
Diabetes was induced in rats (15 groups, 6 rats in each group) by the intraperitoneal (i.p.) injection of streptozocin (STZ) at a dose of 20 mg/kg (b.w.) dissolved in freshly prepared phosphate buffer. Seven days after the injection, the blood glucose levels were measured. Each animal with a blood glucose concentration level above 250 mg/100 cm3 was considered to be diabetic and used in the experiments. To prevent the hypoglycemia, which occurred during the 24 h following the STZ administration, 15 % glucose solution was orally given to the antidiabetic rats. In all experiments, rats were fasted for 6 h prior to STZ injection.
The tested samples 5a–5e, 8a–8e, 10, 12, and glibenclamide (Z) were dissolved DMSO (100 mg/100 cm3) and administered orally (2 mM) solution using a gastric gauge needle. Blood glucose levels were determined after the administration of the tested samples to check the antidiabetic activity of the compounds. Fasting blood glucose level was measured after 7th and 21st day from the animals of all these groups. The blood was collected from the tips of the tail reins and measured using single touch glucometer. These data were expressed in terms of milligram per 100 cm3 of blood and the results are displayed in Table 1.
Toxicity study and determination of LD50
LD50 of the studied compound 8b was determined as described by Olfert et al. [37]. In this experiment, six groups each of 8 male albino mice weighing 20–25 g were used. One group serves as control and other groups of mice were orally administered the tested compound by gastric tube in gradual increasing doses (400, 500, 600, 800, and 1000 mg/kg b.w.). After 48 h of administration, the number of dead animals in each group, the mean of dead animals in two successive doses (z) and the constant factor between two successive doses (d) were recorded and LD50 was calculated as follow: LD50 = D m −Σ (z × d)/n, where D m = the largest dose which kills all animals, z = mean of dead animals between two successive records, d = the constant factor between two successive doses, n = number of animals in each group, Σ = the sum of (z × d).
ABTS antioxidant activity assay
Antioxidant activity determinations were evaluated from the bleaching of ABTS radical cation. The radical cation was derived from ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)], and was prepared by reaction of 60 mm3 ABTS with 3 cm3 MnO2 (25 mg/cm3) in 5 cm3 phosphate buffer solution (10 µM, pH 7). After shaking the solution for a few minutes, it was centrifuged and filtered. The absorbance (A control) of the resulting green–blue solution (ABTS radical solution) was recorded at λ max = 734 nm. The absorbance (A test) was measured upon the addition of 20 mm3 of 1 mg/cm3 solution of the tested sample in spectroscopic grade MeOH/buffer (1:1 v/v) to the ABTS solution. The decrease in the absorbance is expressed: % inhibition = [(A control−A sample)/A sample × 100]. Ascorbic acid (20 cm3, 2 mM) solution was used as the positive control. Blank sample was run using solvent without ABTS (Table 2).
Bleomycin-dependent DNA damage assay
The assay was done according to Aesehashet al. [39] with minor modification. The reaction mixture (0.5 cm3) contained DNA (0.5 mg/cm3), bleomycin sulfate (0.05 mg/cm3), MgCl2 (5 mM), FeCl3 (50 mM), and samples to be tested at different concentrations. l-Ascorbic acid was used as a positive control. The mixture was incubated at 37 °C for 1 h. The reaction was terminated by addition of 0.05 cm3 ethylenediamine-tetraacetic acid (EDTA, 0.1 M). The color was developed by adding 0.5 cm3 thiobarbituric acid (TBA, 1 % v/v) and 0.5 cm3 HCl (25 % v/v), followed by heating at 80 °C for 10 min. After centrifugation, the extent of DNA damage was measured by the increase in absorbance at 532 nm.
References
World Health Organization (2013) Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO Consultation. WHO, Geneva
Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ (1998) Diabetologia 41:904
Kitagawa T, Owada M, Urakami T, Yamauchi K (1998) Clin Paediatr 37:111
Kameswara RB, Giri R, Kesavulu MM, Apparao C (2001) J Ethnopharmacol 74:69
Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ (2005) Clin Pharmacol Ther 77:468
Deshmukh TA, Yadav BV, Badole SL, Bodhankar SL, Dhaneshwar SR (2008) J Appl Biomed 6:81
Troev KD (2006) Chemistry and Application of H-phosphonates. Elsevier, Amsterdam
Majoural JP (2002) In: Bertrand G (ed) New aspects in phosphorus chemistry. Springer, Heidelberg
Ashok M, Holla BS (2007) Phosphorus, Sulfur. Silicon Relat Elem 182:981
Quin LD (2000) A guide to organophosphorus chemistry. Wiley-Interscience, New York
Matin MA, Siddiqui RA (1982) Biochem Pharmac 31:1801
Danetskaia EV, Korzun VN, Ramzaev PV, Shakalova VV (1970) Gig Sanit 35:36
Kleinrok Z, Rajtar G (1979) Acta Physiol Pol 30:289
Schwetlick K (1990) Mechanisms of antioxidant action of phosphite and phosphonite esters. In: Scott G (ed) Mechanism of polymer of degradation and stabillisation. Elsevier Applied Science, London, p 23
Schwetlick K (1983) Pure Appl Chem 55:1629
Da Silva CH, Da Silva VB, Resende J, Rodrigues PF, Bononi FC, Benevenuto CG, Taft CA (2010) J Mol Graph Modell 28:513
Lagunin AA, Gomazkov OA, Filimonov DA, Gureeva TA, Dilakyan EA, Kugaevskaya EV, Elisseeva YE, Solovyeva NI, Poroikov VV (2003) J Med Chem 46:3326
Abdou WM, Bekheit MS (2015) Arabian J Chem. doi:10.1016/j.arabjc.2015.04.014
Abdou WM, Barghash RF (2015) J Pharm Pharmacol 3:153
Abdou WM, Ganoub NA, Sabry E (2015) Monatsh Chem. doi:10.1007/s00706-015-1542-4
Barghash RF, Ganoub NA, Abdou WM (2014) Monatsh Chem 145:1621
Abdou WM, Ganoub NA, Barghash RF (2014) Synth Commun 44:2669
Abdou WM, Ganoub NA, Sabry E (2014) Acta Pharm 64:267
Jansa P, Baszczyňski O, Procházková E, Dračínský M, Janeba Z (2012) Green Chem 14:2282
Neidlein VR, Buseck S (1992) Helv Chim Acta 75:2520
Barendt JM, Bent EG, Haltiwanger RC, Squier CA, Norman AD (1989) Inorg Chem 28:4425
Emsley J, Hall D (1976) The chemistry of phosphorus, vol 2. Harper and Row limited, London, p 29
Kaname M, Sashida H (2012) Tetrahedron Lett 53:748
Zhang W, Chen CHT, Nagashima T (2003) Tetrahedron Lett 44:2065
Kaboudin B, Fallahi M (2011) Tetrahedron Lett 52:4346
Ali TES (2009) Eur J Med Chem 44:4
World Health Organization (1999) Report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Department of non-communicable disease surveillance. WHO/NCD/NCS/99.2, WHO, Geneva
Wild S, Roglic G, Green A, Sicree R, King H (2004) Diabetes Care 27:1047
Cheng D (2005) Nutr Metab 2:29
Islam MS, Loots DT (2009) Methods Find Exp Clin Pharmacol 31:249
Atkin SH, Dasmahapatra A, Jaker MA, Chorost MI, Reddy S (1991) Annu Int Med 114:1020
Olfert ED, Cross BM, McWilliam AA (1993) Guide to the care and use of experimental animals, vol 1. CCAC, Ottawa
Lissi EA, Modak B, Torres R, Esocbar J, Urzua A (1999) Free Radical Res 30:471
Aeschbach R, Löliger J, Scott BC, Murcia A, Buttler J, Halliwell B, Aruoma OI (1994) Food Chem Toxicol 32:31
Pudovik MA, Mikhallov YuB, Pudovik AN (1981) Izv Akad Nauk SSSR Ser Khim 5:1108
Acknowledgments
The authors express their Grateful thanks to the National Research Centre, Dokki, Cairo, Egypt for the financial support, project # 10010101. Authors are also grateful to High Institute of Nutrition and Diabetes, Ministry of High Education, Cairo, Egypt for executing pharmacological screening.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00706-016-1767-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdou, W.M., Bekheit, M.S. & Barghash, R.F. Microwave-assisted synthesis and diabetic/antioxidant assessments of 1,3,2-benzothiazaphosphole-3(2H)-carbothioamide- and -diazaphosphole-3(2H)-dicarbothioamide 2-oxide derivatives. Monatsh Chem 147, 1797–1808 (2016). https://doi.org/10.1007/s00706-016-1721-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1721-y