Abstract
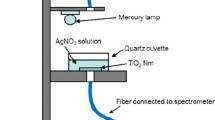
Ag-doped TiO2 (anatase) samples (mass fraction w Ag = 0.01 and w Ag = 0.02) of 15.9 and 14.5 nm mean particle size and 11.46 and 10.14 m2 g−1 BET surface area were prepared by photodeposition. Doping results in surface plasmon resonance of the metallic silver nanoclusters at around 500 nm, but the absorption edge remains unaltered at 365 nm. Ag-doping remarkably enhances the photooxidation of iodide ion under UV light; iodine formation with Ag/TiO2 with w Ag = 0.01 is 16 times greater than with bare TiO2. The reaction conforms to Langmuir–Hinshelwood kinetics with regard to both I− and O2. Increase of pH slows down iodine formation and sacrificial electron donors arrest the reaction. Pre-sonication of the catalyst slurry hinders the photocatalysis. Generation of iodine is much greater in acetonitrile than in water. Under the experimental conditions, Ag/TiO2 with w Ag = 0.01 is more efficient than Ag/TiO2 with w Ag = 0.02, and the enhanced photocatalysis is likely to be because of suppression of electron–hole pair recombination. Kinetic analysis reveals that increasing the Ag mass fraction from 0.01 to 0.02 enhances the surface pseudo-first-order rate constant but inhibits the adsorption of iodide ion and the oxygen molecule on the illuminated oxide surface.
Graphical Abstract














Similar content being viewed by others
References
Thompson TL, Yates JT Jr (2006) Chem Rev 106:4428
Gaya UI, Abdullah AH (2008) J Photochem Photobiol C 9:1
Diebold U (2003) Surf Sci Rep 48:53
Osgood R (2006) Chem Rev 106:4379
Zhao J, Li B, Onda K, Feng M, Petek H (2006) Chem Rev 106:4402
Shiraishi Y, Saito N, Hirai T (2005) J Am Chem Soc 127:12820
Peller J, Wiest O, Kamat PV (2004) J Phys Chem A 108:10925
Du Y, Rabani J (2003) J Phys Chem B 107:11970
Sun L, Bolton JR (1996) J Phys Chem 100:4127
Linsebigler AL, Lu G, Yates JT Jr (1995) Chem Rev 95:735
Bansal A, Madhavi S, Tan TTY, Lim TM (2008) Catal Today 131:250
Young C, Lim TM, Chiang K, Scott J, Amal R (2008) Appl Catal B 78:1
Sa J, Fernandez-Garcia M, Anderson JA (2008) Catal Commun 9:1991
Paramasivam I, Macak JM, Schmuki P (2008) Electrochem Commun 10:71
Seery MK, George R, Floris P, Pillai SC (2007) J Photochem Photobiol A 189:258
Lee MS, Hong SS, Mohseni M (2005) J Mol Catal A 242:135
Sung-Suh HM, Choi JR, Hah HJ, Koo SM, Bae YC (2004) J Photochem Photobiol A 163:37
Trans H, Scott J, Chiang K, Amal R (2006) J Photochem Photobiol A 183:41
Zhang Z, Ito S, Moser JE, Zakeeruddin SM, Gratzel M (2009) ChemPhysChem 10:1834
Green ANM, Chandler RE, Haque SA, Nelson J, Durrant JR (2005) J Phys Chem B 109:142
Fitzmaurice DJ, Eschie M, Frei H (1993) J Phys Chem 97:3806
Karunakaran C, Anilkumar P (2008) Solar Energy Mater Solar Cells 92:490
Karunakaran C, Anilkumar P (2007) J Mol Catal A 265:153
Karunakaran C, Senthilvelan S, Karuthapandian S, Balaraman K (2004) Catal Commun 5:283
Ishibashi K-I, Fujishima A, Watanabe T, Hashimoto K (2000) J Photochem Photobiol A 134:139
Ohno T, Fujihara K, Saito S, Matsumura M (1997) Solar Energy Mater Solar Cells 45:169
Hodak J, Quinteros C, Litter MI, Roman ES (1996) J Chem Soc Faraday Trans 92:5081
Tennakone K, Kumarasinghe AR, Kumara GRRA, Wijayantha KGU, Sirimanne PM (1997) J Photochem Photobiol A 108:193
Zhang L, Yu JC (2005) Catal Commun 6:684
Zhang F, Pi Y, Cui J, Yang Y, Zhang X, Guan N (2007) J Phys Chem C 111:3756
Hirano K, Nitta H, Sawada K (2005) Ultrason Sonochem 12:271
Karunakaran C, Senthilvelan S, Karuthapandian S (2005) J Photochem Photobiol A 172:207
Vincze L, Kemp TJ (1995) J Photochem Photobiol A 87:257
Karunakaran C, Sujatha MP, Gomathisankar P (2009) Monatsh Chem 140:1269
Kuhn HJ, Braslavsky SE, Schmidt R (2004) Pure Appl Chem 76:2105
Acknowledgments
Financial support through research grant no. F.12-64/2003 (SR) by the University Grants Commission (UGC), New Delhi, is thankfully acknowledged, and P.A. is grateful to UGC for PF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karunakaran, C., Anilkumar, P. & Gomathisankar, P. Kinetics of Ag/TiO2-photocatalyzed iodide ion oxidation. Monatsh Chem 141, 529–537 (2010). https://doi.org/10.1007/s00706-010-0288-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0288-2