Abstract
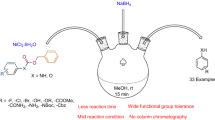
1-Butyl-3-methyl-imidazolium chloride ([bmim]Cl) in the absence of any catalyst mediated the selective deprotection of benzyl and phenyl trimethylsilyl (TMS) ethers to the corresponding alcohols and phenol in good yields at room temperature even in presence of alkyl silyl ethers. The work-up of reactions is very simple and the products do not require further purification. The ionic liquid (IL) can be recycled and reused for several runs without any significant loss of activity.
Similar content being viewed by others
References
TW Greene PGM Wuts (1999) Protective Groups in Organic Synthesis EditionNumber3 Wiley New York
PJ Kocienski (1994) Protecting Groups George Thieme Verlag New York
EJ Corey A Venkateswarlu (1972) J Am Chem Soc 94 6190 Occurrence Handle10.1021/ja00772a043 Occurrence Handle1:CAS:528:DyaE3sXktlGlug%3D%3D
Lalonde M, Chan TH (1985) Synthesis:817
SR Jackson MG Johnson M Mikami S Shiokawa EM Carreira (2001) Angew Chem Intl Ed 40 2694 Occurrence Handle10.1002/1521-3773(20010716)40:14<2694::AID-ANIE2694>3.0.CO;2-D Occurrence Handle1:CAS:528:DC%2BD3MXlsFensLo%3D
Nelson TD, Crouch RD (1996) Synthesis:1031
Yang YY, Yang WB, Teo CF, Lin CH (2000) Synlett:1634
YJ Jeong JH Lee ES Park CM Yoon (2002) J Chem Soc Perkin Trans 1 1223 Occurrence Handle10.1039/b201794f Occurrence Handle1:CAS:528:DC%2BD38XjsFKnsrY%3D
Oriyama T, Kobayashi Y, Noda K (1998) Synlett:1047
JS Yadav BVS Reddy C Madan (2000) New J Chem 24 853 Occurrence Handle10.1039/b004937i Occurrence Handle1:CAS:528:DC%2BD3cXnslSiu7w%3D
RD Crouch JM Polizzi RA Cleiman J Yi CA Romany (2002) Tetrahedron Lett 43 7151 Occurrence Handle10.1016/S0040-4039(02)01680-5 Occurrence Handle1:CAS:528:DC%2BD38Xns12isr0%3D
BC Ranu U Jana A Majee (1999) Tetrahedron Lett 40 1985 Occurrence Handle10.1016/S0040-4039(99)00097-0 Occurrence Handle1:CAS:528:DyaK1MXhvVSlur0%3D
G Bartoli G Cupone R Dalpozzo A De Nino L Maiuolo A Procopio L Sambri A Tararelli (2002) Tetrahedron Lett 43 5945 Occurrence Handle10.1016/S0040-4039(02)01254-6 Occurrence Handle1:CAS:528:DC%2BD38XlvVKisbw%3D
Bartoli G, Bosco M, Marcantoni E, Sambri L, Torregiani E (1998) Synlett:209
R Hunter W Hinz P Richards (1999) Tetrahedron Lett 40 3643 Occurrence Handle10.1016/S0040-4039(99)00523-7 Occurrence Handle1:CAS:528:DyaK1MXivFanur0%3D
RD Crouch CA Romany AC Kreshock KA Menconi JL Zile (2004) Tetrahedron Lett 45 1279 Occurrence Handle10.1016/j.tetlet.2003.11.134 Occurrence Handle1:CAS:528:DC%2BD2cXltFOlsA%3D%3D
PMC Gloria S Prabhakar AM Lobo MJS Gomes (2003) Tetrahedron Lett 44 8819 Occurrence Handle10.1016/j.tetlet.2003.09.191 Occurrence Handle1:CAS:528:DC%2BD3sXos1OgsLs%3D
J Farras C Serra J Vilarrasa (1998) Tetrahedron Lett 39 327 Occurrence Handle10.1016/S0040-4039(97)10479-8 Occurrence Handle1:CAS:528:DyaK1cXltVSgsQ%3D%3D
JS Bajwa J Vivelo J Slade O Repic T Blacklock (2000) Tetrahedron Lett 41 6021 Occurrence Handle10.1016/S0040-4039(00)01012-1 Occurrence Handle1:CAS:528:DC%2BD3cXlt1yrs74%3D
EJ Corey JW Ponder P Ulrich (1980) Tetrahedron Lett 21 137 Occurrence Handle10.1016/S0040-4039(00)71395-5 Occurrence Handle1:CAS:528:DyaL3cXksVKgsL8%3D
KA Scheidt H Chen BC Follows SR Chemler DS Coffey WR Roush (1998) J Org Chem 63 6436 Occurrence Handle10.1021/jo981215i Occurrence Handle1:CAS:528:DyaK1cXls12mtr8%3D
Vaino AR, Szarek WA (1996) J Chem Soc Chem Commun:2351
MT Barros CD Maycock F Sineriz C Thomassigny (2000) Tetrahedron 56 6511 Occurrence Handle10.1016/S0040-4020(00)00593-7 Occurrence Handle1:CAS:528:DC%2BD3cXmtValurg%3D
Barros MT, Maycock CD, Thomassigny C (2001) Synlett:1146
ASY Lee HC Yeh JJ Shie (1998) Tetrahedron Lett 39 5249 Occurrence Handle10.1016/S0040-4039(98)01035-1 Occurrence Handle1:CAS:528:DyaK1cXkt1Kks78%3D
CE Yeom YJ Kim SY Lee YJ Shin BM Kim (2005) Tetrahedron 61 12227 Occurrence Handle10.1016/j.tet.2005.10.043 Occurrence Handle1:CAS:528:DC%2BD2MXht1OisL3N
B Karimi A Zamania D Zareyeeb (2004) Tetrahedron Lett 45 9139 Occurrence Handle10.1016/j.tetlet.2004.09.157 Occurrence Handle1:CAS:528:DC%2BD2cXpsVKjs70%3D
JF Cormier (1991) Tetrahedron Lett 32 187 Occurrence Handle10.1016/0040-4039(91)80850-6 Occurrence Handle1:CAS:528:DyaK3MXhvVGqsrY%3D
EJ Corey GB Jones (1992) J Org Chem 57 1028 Occurrence Handle10.1021/jo00029a050 Occurrence Handle1:CAS:528:DyaK38XlvVymtw%3D%3D
EFJ Devries J Brussee A vandergen (1994) J Org Chem 59 7133 Occurrence Handle10.1021/jo00102a047 Occurrence Handle1:CAS:528:DyaK2MXhslyjtLw%3D
PT Anasta JC Warner (1998) Green Chemistry: Theory and Practice Oxford University Press New York
Anasta PT (2002) In: Abraham MA, Moens L (eds) Clean Solvents, Alternati Ve Media for Chemical Reactions and Processing, ACS Symposium Series 819, American Chemical Society, Washington, DC, p 1
T Welton (1999) Chem Rev 99 2071 Occurrence Handle10.1021/cr980032t Occurrence Handle1:CAS:528:DyaK1MXkt1artrw%3D
P Wasserscheid W Keim (2000) Angew Chem Int Ed 39 3772 Occurrence Handle10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5 Occurrence Handle1:CAS:528:DC%2BD3cXotFyqtLk%3D
Sheldon RA (2001) Chem Commun:2399
RA Rrown P Pollet E Mckoon CA Eckert CL Liotta PG Jessop (2001) J Am Chem Soc 123 1254 Occurrence Handle10.1021/ja005718t Occurrence Handle1:CAS:528:DC%2BD3MXksVOisQ%3D%3D
K Selvakumar A Zapf M Beller (2002) Org Lett 4 3033 Occurrence Handle10.1021/ol020103h Occurrence Handle1:CAS:528:DC%2BD38XmtFSrt7s%3D
J Mo L Xu J Xiao (2005) J Am Chem Soc 127 751 Occurrence Handle10.1021/ja0450861 Occurrence Handle1:CAS:528:DC%2BD2cXhtVyltrvK
A Shaabani AH Rezayan (2005) J Porphyr Phthalocya 9 617 Occurrence Handle1:CAS:528:DC%2BD28XhslOnu7s%3D Occurrence Handle10.1142/S108842460500071X
KE Johnson RM Pagni J Bartmess (2007) Monatsh Chem 138 1077 Occurrence Handle10.1007/s00706-007-0755-6 Occurrence Handle1:CAS:528:DC%2BD2sXht1Gju7zI
JM Leveque J Estager M Draye G Cravotto L Boffa W Bonrath (2007) Monatsh Chem 138 1103 Occurrence Handle10.1007/s00706-007-0742-y Occurrence Handle1:CAS:528:DC%2BD2sXht1Gju7rP
A Shaabani E Soleimani A Maleki (2006) Tetrahedron Lett 47 3031 Occurrence Handle10.1016/j.tetlet.2006.03.011 Occurrence Handle1:CAS:528:DC%2BD28XjtFCqt7s%3D
A Shaabani A Sarvary A Rahmati AH Rezayan (2007) Lett Org Chem 4 68 Occurrence Handle10.2174/157017807780037531 Occurrence Handle1:CAS:528:DC%2BD2sXjtlCjs78%3D
A Shaabani AH Rezayan A Rahmati A Rahmati M Sharifi (2006) Monatsh Chem 138 77 Occurrence Handle10.1007/s00706-005-0405-9 Occurrence Handle1:CAS:528:DC%2BD2MXhtlCqu7zF
B Karimi B Golshani (2000) J Org Chem 65 7228 Occurrence Handle10.1021/jo005519s Occurrence Handle1:CAS:528:DC%2BD3cXmslGju70%3D
BE Blass CL Harris DE Portlock (2001) Tetrahedron Lett 42 1611 Occurrence Handle10.1016/S0040-4039(00)02259-0 Occurrence Handle1:CAS:528:DC%2BD3MXht1yrtrw%3D
Author information
Authors and Affiliations
Corresponding author
Additional information
Correspondence: Ahmad Shaabani, Department of Chemistry, Shahid Beheshti University, PO Box 19396-4716, Tehran, Iran.
Rights and permissions
About this article
Cite this article
Shaabani, A., Rezayan, A., Heidary, M. et al. A mild and efficient approach for the selective deprotection of benzyl and phenyl trimethylsilyl ethers in 1-butyl-3-methyl-imidazolium chloride. Monatsh Chem 139, 1471–1474 (2008). https://doi.org/10.1007/s00706-008-0960-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-008-0960-y