Abstract
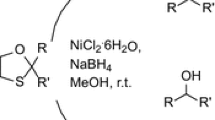
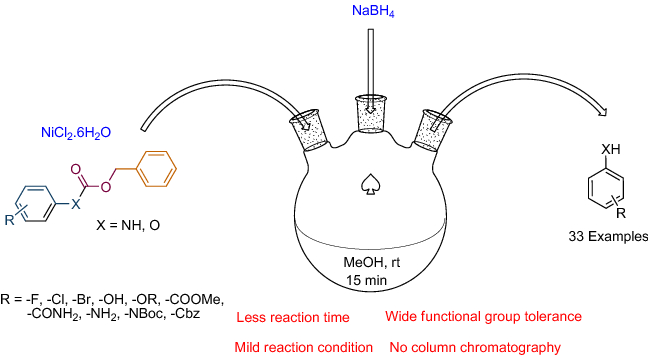
A novel protocol for the deprotection of N-benzyloxycarbonyl and O-benzyloxycarbonyl groups by nickel boride generated in situ from NaBH4 and NiCl2·6H2O in methanol at room temperature has been developed to give the corresponding amines and phenols. This protocol is chemoselective as groups like chloro, bromo, amide, ester, pyridine, and tert-butyloxycarbonyl moiety are unaffected under these conditions. The deprotection has also been validated in gram scale reactions, to establish the wider appropriateness of this protocol.
Graphical abstract
Similar content being viewed by others
References
Greene TW, Wuts PGM (1999) Protective groups in organic synthesis, 3rd edn. Wiley, New York
Jarowicki K, Kocienski PJ (1999) J Chem Soc Perkin Trans 1:1589
Jarowicki K, Kocienski PJ (2000) J Chem Soc Perkin Trans 1:2495
Sakaitani M, Ohfune Y (1990) J Org Chem 55:870
Coleman RS, Carpenter AJ (1992) J Org Chem 57:5813
Mao L, Wang Z, Li Y, Han X, Zhou W (2011) Synlett:129
Watkins BE, Kiely JS, Rapoport H (1982) J Am Chem Soc 104:5702
Lawrence SA (2004) Amines: synthesis, Properties and Applications. Cambridge University Press, Cambridge
Johnson DC, Widlanski TS (2004) Org Lett 25:4643
Carato P, Yous S, Sellier D, Poupaert JH, Lebeguea N, Berthelot P (2004) Tetrahedron 60:10321
Behloul C, Guijarro D, Yus M (2005) Tetrahedron 61:9319
Felix AM (1974) J Org Chem 39:1427
Schulhof JC, Molko D, Teoule R (1987) Nucleic Acids Res 15:397
Satyanarayana K, Chidambaram N, Chandrasekaran S (1989) Synth Commun 19:2159
Vatèle JM (2004) Tetrahedron 19:4251
Anderson WK, Kinder FR (1990) J Heterocycl Chem 27:975
Khurana JM, Ray A, Singh S (1998) Tetrahedron Lett 39:3829
Khurana JM, Kandpal BM, Kukreja G, Sharma P (2006) Can J Chem 84:1019
Khurana JM, Sharma P (2004) Bull Chem Soc Jpn 77:549
Khurana JM, Agrawal A, Kukreja G (2006) Heterocycles 68:1885
Khurana JM, Arora R (2009) Synthesis 2009:1127
Khurana JM, Arora R, Satija S (2007) Heterocycles 71:2709
Khurana JM, Gogia A (1997) Org Prep Proced Int 29:1
Khurana JM, Kukreja G, Bansal G (2002) J Chem Soc Perkin Trans 1:2520
Khurana JM, Dawra K, Sharma P (2015) RSC Adv 5:12048
Khurana JM, Magoo D, Dawra K (2016) Monatsh Chem 147:1113
Bartwal G, Saroha M, Khurana JM (2018) Synth Commun 48:97
Acknowledgements
M. S. and G. B. thank CSIR, New Delhi, India for the Grant of SPM Fellowship and JRF and SRF, respectively. Authors also acknowledge University of Delhi for providing us University Grant and DST Purse Grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saroha, M., Aggarwal, K., Bartwal, G. et al. Development of a novel protocol for chemoselective deprotection of N/O-benzyloxycarbonyl (Cbz) at ambient temperature. Monatsh Chem 149, 2231–2235 (2018). https://doi.org/10.1007/s00706-018-2276-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2276-x