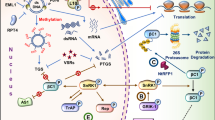
Abstract
Geminiviruses have mastered plant cell modulation and immune invasion to ensue prolific infection. Encoding a relatively small number of multifunctional proteins, geminiviruses rely on satellites to efficiently re-wire plant immunity, thereby fostering virulence. Among the known satellites, betasatellites have been the most extensively investigated. They contribute significantly to virulence, enhance virus accumulation, and induce disease symptoms. To date, only two betasatellite proteins, βC1, and βV1, have been shown to play a crucial role in virus infection. In this review, we offer an overview of plant responses to betasatellites and counter-defense strategies deployed by betasatellites to overcome those responses.



Similar content being viewed by others
References
Brito AF, Pinney JW (2017) Protein–Protein Interactions in Virus–Host Systems. Front Microbiol 8:
R KC, Ali Z, M TT, et al (2018) Hacking the Cell: Network Intrusion and Exploitation by Adenovirus E1A. mBio 9:e00390-18. https://doi.org/10.1128/mBio.00390-18
Hanley-Bowdoin L, Bejarano ER, Robertson D, Mansoor S (2013) Geminiviruses: masters at redirecting and reprogramming plant processes. Nat Rev Microbiol 11:777–788. https://doi.org/10.1038/nrmicro3117
Gong P, Tan H, Zhao S, et al (2021) Geminiviruses encode additional small proteins with specific subcellular localizations and virulence function. Nat Commun 12:4278. https://doi.org/10.1038/s41467-021-24617-4
Gupta N, Reddy K, Bhattacharyya D, Chakraborty S (2021) Plant responses to geminivirus infection: guardians of the plant immunity. Virol J 18:143. https://doi.org/10.1186/s12985-021-01612-1
Rojas M, Macedo M, Maliano M, et al (2018) World Management of Geminiviruses. Annu Rev Phytopathol 56:637–677. https://doi.org/10.1146/annurev-phyto-080615-100327
Devendran R, Kumar M, Ghosh D, et al (2022) Capsicum-infecting begomoviruses as global pathogens: host–virus interplay, pathogenesis, and management. Trends Microbiol 30:170–184. https://doi.org/10.1016/j.tim.2021.05.007
Zerbini FM, Briddon RW, Idris A, et al (2017) ICTV Virus Taxonomy Profile: Geminiviridae. Journal of General Virology 98:131–133. https://doi.org/10.1099/jgv.0.000738
Fiallo-Olivé E, Lett J-M, Martin D, et al (2021) ICTV Virus Taxonomy Profile: Geminiviridae 2021. Journal of General Virology 102:. https://doi.org/10.1099/jgv.0.001696
Blanc S, Gutiérrez S (2015) The specifics of vector transmission of arboviruses of vertebrates and plants. Curr Opin Virol 15:27–33. https://doi.org/10.1016/j.coviro.2015.07.003
Varsani A, Roumagnac P, Fuchs M, et al (2017) Capulavirus and Grablovirus: two new genera in the family Geminiviridae. Arch Virol 162:1819–1831. https://doi.org/10.1007/s00705-017-3268-6
Kishorekumar R, Devendran R, Chakraborty S (2022) Insights into the multifunctional roles of geminivirusencoded proteins in pathogenesis. Arch Virol. https://doi.org/10.1007/s00705-021-05338-x
Dry IB, Krake LR, Rigden JE, Rezaian MA (1997) A novel subviral agent associated with a geminivirus: The first report of a DNA satellite. Proceedings of the National Academy of Sciences 94:7088–7093. https://doi.org/10.1073/pnas.94.13.7088
Saunders K, Bedford I, Stanley J (2002) Adaptation from whitefly to leafhopper transmission of an autonomously replicating nanovirus-like DNA component associated with ageratum yellow vein disease. J Gen Virol 83:907–913. https://doi.org/10.1099/0022-1317-83-4-907
Mansoor S, Zafar Y, Briddon RW (2006) Geminivirus disease complexes: the threat is spreading. Trends Plant Sci 11:209–212. https://doi.org/10.1016/j.tplants.2006.03.003
Nawaz-ul-Rehman MS, Fauquet CM (2009) Evolution of geminiviruses and their satellites. FEBS Lett 583:1825–1832. https://doi.org/10.1016/j.febslet.2009.05.045
Kumar RV, Singh AK, Singh AK, et al (2015) Complexity of begomovirus and betasatellite populations associated with chilli leaf curl disease in India. Journal of General Virology 96:3143–3158. https://doi.org/10.1099/jgv.0.000254
Vinoth Kumar R, Singh D, Singh AK, Chakraborty S (2017) Molecular diversity, recombination and population structure of alphasatellites associated with begomovirus disease complexes. Infection, Genetics and Evolution 49:39–47. https://doi.org/10.1016/j.meegid.2017.01.001
Xiaofeng C, Guixin L, Daowen W, et al (2005) A Begomovirus DNAβ-Encoded Protein Binds DNA, Functions as a Suppressor of RNA Silencing, and Targets the Cell Nucleus. J Virol 79:10764–10775. https://doi.org/10.1128/JVI.79.16.10764-10775.2005
Saunders K, Briddon RW, Stanley J (2008) Replication promiscuity of DNA-β satellites associated with monopartite begomoviruses; deletion mutagenesis of the Ageratum yellow vein virus DNA-β satellite localizes sequences involved in replication. Journal of General Virology 89:3165–3172. https://doi.org/10.1099/vir.0.2008/003848-0
Idris A, Shahid MS, Briddon R, et al (2010) An unusual alphasatellite associated with monopartite begomoviruses attenuates symptoms and reduces betasatellite accumulation. J Gen Virol 92:706–717. https://doi.org/10.1099/vir.0.025288-0
Nawaz-ul-Rehman MS, Nahid N, Mansoor S, et al (2010) Post-transcriptional gene silencing suppressor activity of two non-pathogenic alphasatellites associated with a begomovirus. Virology 405:300–308. https://doi.org/10.1016/j.virol.2010.06.024
Kumar M, Zarreen F, Chakraborty S (2021) Roles of two distinct alphasatellites modulating geminivirus pathogenesis. Virol J 18:249. https://doi.org/10.1186/s12985-021-01718-6
Zhao L, Che X, Wang Z, et al (2022) Functional Characterization of Replication-Associated Proteins Encoded by Alphasatellites Identified in Yunnan Province, China. Viruses 14:222. https://doi.org/10.3390/v14020222
Fiallo-Olivé E, Martínez-Zubiaur Y, Moriones E, Navas-Castillo J (2012) A novel class of DNA satellites associated with New World begomoviruses. Virology 426:1–6. https://doi.org/10.1016/j.virol.2012.01.024
Fiallo-Olivé E, Tovar R, Navas-Castillo J (2016) Deciphering the biology of deltasatellites from the New World: maintenance by New World begomoviruses and whitefly transmission. New Phytologist 212:680–692. https://doi.org/10.1111/nph.14071
Hassan I, Orílio AF, Fiallo-Olivé E, et al (2016) Infectivity, effects on helper viruses and whitefly transmission of the deltasatellites associated with sweepoviruses (genus Begomovirus, family Geminiviridae). Sci Rep 6:30204. https://doi.org/10.1038/srep30204
Sivalingam PN, Varma A (2012) Role of betasatellite in the pathogenesis of a bipartite begomovirus affecting tomato in India. Arch Virol 157:1081–1092. https://doi.org/10.1007/s00705-012-1261-7
Zhou X (2013) Advances in Understanding Begomovirus Satellites. Annu Rev Phytopathol 51:357–381. https://doi.org/10.1146/annurev-phyto-082712-102234
Li F, Yang X, Bisaro DM, Zhou X (2018) The βC1 Protein of Geminivirus–Betasatellite Complexes: A Target and Repressor of Host Defenses. Mol Plant 11:1424–1426. https://doi.org/10.1016/j.molp.2018.10.007
Gnanasekaran P, Chakraborty S (2018) Biology of viral satellites and their role in pathogenesis. Curr Opin Virol 33:96–105. https://doi.org/10.1016/j.coviro.2018.08.002
Mansoor S, Khan SH, Bashir A, et al (1999) Identification of a Novel Circular Single-Stranded DNA Associated with Cotton Leaf Curl Disease in Pakistan. Virology 259:190–199. https://doi.org/10.1006/viro.1999.9766
Yang X, Xie Y, Raja P, et al (2011) Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection. PLoS Pathog 7:e1002329-
Li F, Huang C, Li Z, Zhou X (2014) Suppression of RNA Silencing by a Plant DNA Virus Satellite Requires a Host Calmodulin-Like Protein to Repress RDR6 Expression. PLoS Pathog 10:e1003921-
Bhattacharyya D, Gnanasekaran P, Kumar RK, et al (2015) A geminivirus betasatellite damages the structural and functional integrity of chloroplasts leading to symptom formation and inhibition of photosynthesis. J Exp Bot 66:5881–5895. https://doi.org/10.1093/jxb/erv299
Xueting Z, Qi WZ, Ruyuan X, et al (2017) Mimic Phosphorylation of a βC1 Protein Encoded by TYLCCNB Impairs Its Functions as a Viral Suppressor of RNA Silencing and a Symptom Determinant. J Virol 91:e00300-17. https://doi.org/10.1128/JVI.00300-17
Yang X, Guo W, Li F, et al (2019) Geminivirus-Associated Betasatellites: Exploiting Chinks in the Antiviral Arsenal of Plants. Trends Plant Sci 24:519–529. https://doi.org/10.1016/j.tplants.2019.03.010
Prabu G, Neha G, Kalaiarasan P, Supriya C (2021) Geminivirus Betasatellite-Encoded βC1 Protein Exhibits Novel ATP Hydrolysis Activity That Influences Its DNA-Binding Activity and Viral Pathogenesis. J Virol 95:e00475-21. https://doi.org/10.1128/JVI.00475-21
King AMQ, Lefkowitz EJ, Mushegian AR, et al (2018) Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Arch Virol 163:2601–2631. https://doi.org/10.1007/s00705-018-3847-1
Wang N, Zhao P, Wang D, et al (2022) Diverse Begomoviruses Evolutionarily Hijack Plant Terpenoid-Based Defense to Promote Whitefly Performance. Cells 12:149. https://doi.org/10.3390/cells12010149
Hu T, Song Y, Wang Y, Zhou X (2020) Functional analysis of a novel βV1 gene identified in a geminivirus betasatellite. Sci China Life Sci 63:688–696. https://doi.org/10.1007/s11427-020-1654-x
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329. https://doi.org/10.1038/nature05286
Ngou BPM, Ding P, Jones JDG (2022) Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 34:1447–1478. https://doi.org/10.1093/plcell/koac041
Wang Y, Pruitt RN, Nürnberger T, Wang Y (2022) Evasion of plant immunity by microbial pathogens. Nat Rev Microbiol 20:449–464. https://doi.org/10.1038/s41579-022-00710-3
Haasnoot J, Westerhout EM, Berkhout B (2007) RNA interference against viruses: strike and counterstrike. Nat Biotechnol 25:1435–1443. https://doi.org/10.1038/nbt1369
Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833. https://doi.org/10.1038/35081161
Rao M v, Lee H, Creelman RA, et al (2000) Jasmonic Acid Signaling Modulates Ozone-Induced Hypersensitive Cell Death. Plant Cell 12:1633–1646. https://doi.org/10.1105/tpc.12.9.1633
O’Brien JA, Daudi A, Butt VS, Paul Bolwell G (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236:765–779. https://doi.org/10.1007/s00425-012-1696-9
Fu ZQ, Yan S, Saleh A, et al (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232. https://doi.org/10.1038/nature11162
Xu J, Zhang S (2015) Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci 20:56–64. https://doi.org/10.1016/j.tplants.2014.10.001
Gupta N, Reddy K, Gnanasekaran P, et al (2022) Functional characterization of a new ORF βV1 encoded by radish leaf curl betasatellite. Front Plant Sci 13:
Shen Q, Liu Z, Song F, et al (2011) Tomato SlSnRK1 Protein Interacts with and Phosphorylates βC1, a Pathogenesis Protein Encoded by a Geminivirus β-Satellite. Plant Physiol 157:1394–1406. https://doi.org/10.1104/pp.111.184648
Shen Q, Hu T, Bao M, et al (2016) Tobacco RING E3 Ligase NtRFP1 Mediates Ubiquitination and Proteasomal Degradation of a Geminivirus-Encoded βC1. Mol Plant 9:911–925. https://doi.org/10.1016/j.molp.2016.03.008
Haxim Y, Ismayil A, Jia Q, et al (2017) Autophagy functions as an antiviral mechanism against geminiviruses in plants. Elife 6:e23897. https://doi.org/10.7554/eLife.23897
Ismayil A, Yang M, Haxim Y, et al (2020) Cotton leaf curl Multan virus βC1 Protein Induces Autophagy by Disrupting the Interaction of Autophagy-Related Protein 3 with Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Cell 32:tpc.00759.2019. https://doi.org/10.1105/tpc.19.00759
Macho AP, Zipfel C (2014) Plant PRRs and the Activation of Innate Immune Signaling. Mol Cell 54:263–272. https://doi.org/10.1016/j.molcel.2014.03.028
Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 10:339–346. https://doi.org/10.1016/j.tplants.2005.05.009
Meng X, Zhang S (2013) MAPK Cascades in Plant Disease Resistance Signaling. Annu Rev Phytopathol 51:245–266. https://doi.org/10.1146/annurev-phyto-082712-102314
Bi G, Zhou J-M (2017) MAP Kinase Signaling Pathways: A Hub of Plant-Microbe Interactions. Cell Host Microbe 21:270–273. https://doi.org/10.1016/j.chom.2017.02.004
Qiu J-L, Zhou L, Yun B-W, et al (2008) Arabidopsis Mitogen-Activated Protein Kinase Kinases MKK1 and MKK2 Have Overlapping Functions in Defense Signaling Mediated by MEKK1, MPK4, and MKS1. Plant Physiol 148:212–222. https://doi.org/10.1104/pp.108.120006
Gao M, Liu J, Bi D, et al (2008) MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 18:1190–1198. https://doi.org/10.1038/cr.2008.300
Kong Q, Qu N, Gao M, et al (2012) The MEKK1-MKK1/MKK2-MPK4 Kinase Cascade Negatively Regulates Immunity Mediated by a Mitogen-Activated Protein Kinase Kinase Kinase in Arabidopsis. Plant Cell 24:2225–2236. https://doi.org/10.1105/tpc.112.097253
Asai T, Tena G, Plotnikova J, et al (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977–983. https://doi.org/10.1038/415977a
Bethke G, Unthan T, Uhrig JF, et al (2009) Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proceedings of the National Academy of Sciences 106:8067–8072. https://doi.org/10.1073/pnas.0810206106
Hu T, Huang C, He Y, et al (2019) βC1 protein encoded in geminivirus satellite concertedly targets MKK2 and MPK4 to counter host defense. PLoS Pathog 15:e1007728-
Wang M-B, Masuta C, Smith NA, Shimura H (2012) RNA Silencing and Plant Viral Diseases. Molecular Plant-Microbe Interactions® 25:1275–1285. https://doi.org/10.1094/MPMI-04-12-0093-CR
Pumplin N, Voinnet O (2013) RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat Rev Microbiol 11:745–760. https://doi.org/10.1038/nrmicro3120
Waterhouse PM, Wang M-B, Lough T (2001) Gene silencing as an adaptive defence against viruses. Nature 411:834–842. https://doi.org/10.1038/35081168
Pallás V, García J (2011) How do plant viruses induce disease? Interactions and interference with host components. J Gen Virol 92:2691–2705. https://doi.org/10.1099/vir.0.034603-0
Wang M-B, Metzlaff M (2005) RNA silencing and antiviral defense in plants. Curr Opin Plant Biol 8:216–222. https://doi.org/10.1016/j.pbi.2005.01.006
Wang M-B, Masuta C, Smith NA, Shimura H (2012) RNA Silencing and Plant Viral Diseases. Molecular Plant-Microbe Interactions® 25:1275–1285. https://doi.org/10.1094/MPMI-04-12-0093-CR
Lindbo JA, Silva-Rosales L, Proebsting WM, Dougherty WG (1993) Induction of a Highly Specific Antiviral State in Transgenic Plants: Implications for Regulation of Gene Expression and Virus Resistance. Plant Cell 5:1749–1759. https://doi.org/10.1105/tpc.5.12.1749
Xiao B, Yang X, Ye C-Y, et al (2014) A diverse set of miRNAs responsive to begomovirus-associated betasatellite in Nicotiana benthamiana. BMC Plant Biol 14:60. https://doi.org/10.1186/1471-2229-14-60
Wang J, Tang Y, Yang Y, et al (2016) Cotton Leaf Curl Multan Virus-Derived Viral Small RNAs Can Target Cotton Genes to Promote Viral Infection. Front Plant Sci 7:
Rishishwar R, Dasgupta I (2019) Suppressors of RNA silencing encoded by geminiviruses and associated DNA satellites. Virusdisease 30:58–65. https://doi.org/10.1007/s13337-018-0418-8
Sunter G, Sunter JL, Bisaro DM (2001) Plants Expressing Tomato Golden Mosaic Virus AL2 or Beet Curly Top Virus L2 Transgenes Show Enhanced Susceptibility to Infection by DNA and RNA Viruses. Virology 285:59–70. https://doi.org/10.1006/viro.2001.0950
Xiangli D, Rene van W, John S, Yiguo H (2003) Functional Characterization of the Nuclear Localization Signal for a Suppressor of Posttranscriptional Gene Silencing. J Virol 77:7026–7033. https://doi.org/10.1128/JVI.77.12.7026-7033.2003
Ramachandran V, Padmanabhan C, S PJ, M FC (2004) Differential Roles of AC2 and AC4 of Cassava Geminiviruses in Mediating Synergism and Suppression of Posttranscriptional Gene Silencing. J Virol 78:9487–9498. https://doi.org/10.1128/JVI.78.17.9487-9498.2004
Daniela T, Rajeswaran R, Shivaprasad P v, et al (2005) Suppression of RNA Silencing by a Geminivirus Nuclear Protein, AC2, Correlates with Transactivation of Host Genes. J Virol 79:2517–2527. https://doi.org/10.1128/JVI.79.4.2517-2527.2005
Wang Y, Gong Q, Wu Y, et al (2021) A calmodulin-binding transcription factor links calcium signaling to antiviral RNAi defense in plants. Cell Host Microbe 29:1393–1406.e7. https://doi.org/10.1016/j.chom.2021.07.003
Mubin M, Ijaz S, Nahid N, et al (2020) Journey of begomovirus betasatellite molecules: from satellites to indispensable partners. Virus Genes 56:16–26. https://doi.org/10.1007/s11262-019-01716-5
Gui X, Liu C, Qi Y, Zhou X (2022) Geminiviruses employ host DNA glycosylases to subvert DNA methylation-mediated defense. Nat Commun 13:575. https://doi.org/10.1038/s41467-022-28262-3
Voorburg CM, Yan Z, Bergua-Vidal M, et al (2020) Ty-1, a universal resistance gene against geminiviruses that is compromised by co-replication of a betasatellite. Mol Plant Pathol 21:160–172. https://doi.org/10.1111/mpp.12885
Briddon RW, Mansoor S, Bedford ID, et al (2001) Identification of DNA Components Required for Induction of Cotton Leaf Curl Disease. Virology 285:234–243. https://doi.org/10.1006/viro.2001.0949
Amin I, Hussain K, Akbergenov R, et al (2011) Suppressors of RNA Silencing Encoded by the Components of the Cotton Leaf Curl Begomovirus-BetaSatellite Complex. Molecular Plant-Microbe Interactions® 24:973–983. https://doi.org/10.1094/MPMI-01-11-0001
Tiwari N, Sharma PK, Malathi VG (2013) Functional characterization of βC1 gene of Cotton leaf curl Multan betasatellite. Virus Genes 46:111–119. https://doi.org/10.1007/s11262-012-0828-4
Sahu AK, Sanan-Mishra N (2021) Interaction between βC1 of satellite and coat protein of Chili leaf curl virus plays a crucial role in suppression of host RNA silencing. Appl Microbiol Biotechnol 105:8329–8342. https://doi.org/10.1007/s00253-021-11624-0
Eini O (2017) A betasatellite-encoded protein regulates key components of gene silencing system in plants. Mol Biol 51:579–585. https://doi.org/10.1134/S0026893317030037
Smalle J, Vierstra RD (2004) THE UBIQUITIN 26S PROTEASOME PROTEOLYTIC PATHWAY. Annu Rev Plant Biol 55:555–590. https://doi.org/10.1146/annurev.arplant.55.031903.141801
Sadanandom A, Bailey M, Ewan R, et al (2012) The ubiquitin–proteasome system: central modifier of plant signalling. New Phytologist 196:13–28. https://doi.org/10.1111/j.1469-8137.2012.04266.x
Vierstra RD (2009) The ubiquitin–26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10:385–397. https://doi.org/10.1038/nrm2688
Üstün S, Sheikh A, Gimenez-Ibanez S, et al (2016) The Proteasome Acts as a Hub for Plant Immunity and Is Targeted by Pseudomonas Type III Effectors. Plant Physiol 172:1941–1958. https://doi.org/10.1104/pp.16.00808
Alcaide-Loridan C, Jupin I (2012) Ubiquitin and Plant Viruses, Let’s Play Together! Plant Physiol 160:72–82. https://doi.org/10.1104/pp.112.201905
Eini O, Dogra S, Selth LA, et al (2009) Interaction with a Host Ubiquitin-Conjugating Enzyme Is Required for the Pathogenicity of a Geminiviral DNA β Satellite. Molecular Plant-Microbe Interactions® 22:737–746. https://doi.org/10.1094/MPMI-22-6-0737
Jia Q, Liu N, Xie K, et al (2016) CLCuMuB βC1 Subverts Ubiquitination by Interacting with NbSKP1s to Enhance Geminivirus Infection in Nicotiana benthamiana. PLoS Pathog 12:e1005668-
Zhou T, Zhang M, Gong P, et al (2021) Selective autophagic receptor NbNBR1 prevents NbRFP1-mediated UPS-dependent degradation of βC1 to promote geminivirus infection. PLoS Pathog 17:e1009956-
Zhang M, Cao B, Zhang H, et al (2022) Geminivirus satellite-encoded βC1 activates UPR, induces bZIP60 nuclear export, and manipulates the expression of bZIP60 downstream genes to benefit virus infection. Sci China Life Sci. https://doi.org/10.1007/s11427-022-2196-y
Islam W, Naveed H, Zaynab M, et al (2019) Plant defense against virus diseases; growth hormones in highlights. Plant Signal Behav 14:1596719. https://doi.org/10.1080/15592324.2019.1596719
Ma K-W, Ma W (2016) Phytohormone pathways as targets of pathogens to facilitate infection. Plant Mol Biol 91:713–725. https://doi.org/10.1007/s11103-016-0452-0
Ghosh D, Chakraborty S (2021) Molecular interplay between phytohormones and geminiviruses: a saga of a never-ending arms race. J Exp Bot 72:2903–2917. https://doi.org/10.1093/jxb/erab061
Bhattacharyya D, Chakraborty S (2018) Chloroplast: the Trojan horse in plant–virus interaction. Mol Plant Pathol 19:504–518. https://doi.org/10.1111/mpp.12533
Gnanasekaran P, Ponnusamy K, Chakraborty S (2019) A geminivirus betasatellite encoded βC1 protein interacts with PsbP and subverts PsbP-mediated antiviral defence in plants. Mol Plant Pathol 20:943–960. https://doi.org/10.1111/mpp.12804
Nair A, Harshith CY, Narjala A, Shivaprasad P V (2023) Begomoviral βC1 orchestrates organellar genomic instability to augment viral infection. The Plant Journal n/a: https://doi.org/10.1111/tpj.16186
Sun Y-C, Pan L-L, Ying F-Z, et al (2017) Jasmonic acid-related resistance in tomato mediates interactions between whitefly and whitefly-transmitted virus. Sci Rep 7:566. https://doi.org/10.1038/s41598-017-00692-w
Yang J-Y, Iwasaki M, Machida C, et al (2008) C1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev 22:2564–2577. https://doi.org/10.1101/gad.1682208
ZHANG T, LUAN J-B, QI J-F, et al (2012) Begomovirus–whitefly mutualism is achieved through repression of plant defences by a virus pathogenicity factor. Mol Ecol 21:1294–1304. https://doi.org/10.1111/j.1365-294X.2012.05457.x
Stone SL, Hauksdóttir H, Troy A, et al (2005) Functional Analysis of the RING-Type Ubiquitin Ligase Family of Arabidopsis. Plant Physiol 137:13–30. https://doi.org/10.1104/pp.104.052423
Li R, Weldegergis BT, Li J, et al (2014) Virulence Factors of Geminivirus Interact with MYC2 to Subvert Plant Resistance and Promote Vector Performance. Plant Cell 26:4991–5008. https://doi.org/10.1105/tpc.114.133181
Pascal E, Sanderfoot AA, Ward BM, et al (1994) The geminivirus BR1 movement protein binds single-stranded DNA and localizes to the cell nucleus. Plant Cell 6:995–1006. https://doi.org/10.1105/tpc.6.7.995
Stefan H, Christina W, Holger J (2004) Interaction of DNA with the Movement Proteins of Geminiviruses Revisited. J Virol 78:7698–7706. https://doi.org/10.1128/JVI.78.14.7698-7706.2004
Noueiry AO, Lucas WJ, Gilbertson RL (1994) Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76:925–932. https://doi.org/10.1016/0092-8674(94)90366-2
Lazarowitz SG, Beachy RN (1999) Viral Movement Proteins as Probes for Intracellular and Intercellular Trafficking in Plants. Plant Cell 11:535–548. https://doi.org/10.1105/tpc.11.4.535
Gafni Y, Epel BL (2002) The role of host and viral proteins in intra- and inter-cellular trafficking of geminiviruses. Physiol Mol Plant Pathol 60:231–241. https://doi.org/10.1006/pmpp.2002.0402
Yanchen Z, R RM, Mi-Ri P, et al (2011) Histone H3 Interacts and Colocalizes with the Nuclear Shuttle Protein and the Movement Protein of a Geminivirus. J Virol 85:11821–11832. https://doi.org/10.1128/JVI.00082-11
Gouveia-Mageste BC, Martins LGC, Dal-Bianco M, et al (2021) A plant-specific syntaxin-6 protein contributes to the intracytoplasmic route for the begomovirus CabLCV. Plant Physiol 187:158–173. https://doi.org/10.1093/plphys/kiab252
Rojas MR, Jiang H, Salati R, et al (2001) Functional Analysis of Proteins Involved in Movement of the Monopartite Begomovirus, Tomato Yellow Leaf Curl Virus. Virology 291:110–125. https://doi.org/10.1006/viro.2001.1194
Gong P, Zhao S, Liu H, et al (2022) Tomato yellow leaf curl virus V3 protein traffics along microfilaments to plasmodesmata to promote virus cell-to-cell movement. Sci China Life Sci 65:1046–1049. https://doi.org/10.1007/s11427-021-2063-4
Li H, Li F, Zhang M, et al (2020) Dynamic Subcellular Localization, Accumulation, and Interactions of Proteins From Tomato Yellow Leaf Curl China Virus and Its Associated Betasatellite. Front Plant Sci 11:
Saeed M, Zafar Y, Randles J, Rezaian M (2007) A monopartite Begomovirus-associated DNA β satellite substitutes for the DNA B of a bipartite begomovirus to permit systemic infection. J Gen Virol 88:2881–2889. https://doi.org/10.1099/vir.0.83049-0
Patil B, Fauquet C (2010) Differential interaction between cassava mosaic geminiviruses and geminivirus satellites. J Gen Virol 91:1871–1882. https://doi.org/10.1099/vir.0.019513-0
Kumar P. P, Usha R, Zrachya A, et al (2006) Protein–protein interactions and nuclear trafficking of coat protein and βC1 protein associated with Bhendi yellow vein mosaic disease. Virus Res 122:127–136. https://doi.org/10.1016/j.virusres.2006.07.007
Nasim A, Rashid MAR, Hussain K, et al (2022) Interaction estimation of pathogenicity determinant protein βC1 encoded by Cotton leaf curl Multan Betasatellite with Nicotiana benthamiana Nuclear Transport Factor 2. PeerJ 10:e14281. https://doi.org/10.7717/peerj.14281
Kamal H, Minhas F ul AA, Tripathi D, et al (2019) βC1, pathogenicity determinant encoded by Cotton leaf curl Multan betasatellite, interacts with calmodulin-like protein 11 (Gh-CML11) in Gossypium hirsutum. PLoS One 14:e0225876. https://doi.org/10.1371/journal.pone.0225876
GÁLIS I, GAQUEREL E, PANDEY SP, BALDWIN IANT (2009) Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ 32:617–627. https://doi.org/10.1111/j.1365-3040.2008.01862.x
Guo Y, Jia M, Li S, Li F (2022) Geminiviruses boost active DNA demethylation for counter-defense. Trends Microbiol 30:1121–1124. https://doi.org/10.1016/j.tim.2022.02.002
Zhao P, Yao X, Cai C, et al (2019) Viruses mobilize plant immunity to deter nonvector insect herbivores. Sci Adv 5:eaav9801. https://doi.org/10.1126/sciadv.aav9801
Naqvi AR, Choudhury NR, Mukherjee SK, Haq QMohdR (2011) In silico analysis reveals that several tomato microRNA/microRNA∗ sequences exhibit propensity to bind to tomato leaf curl virus (ToLCV) associated genomes and most of their encoded open reading frames (ORFs). Plant Physiology and Biochemistry 49:13–17. https://doi.org/10.1016/j.plaphy.2010.09.013
Akhtar S, Tahir N, Amin I, Mansoor S (2021) Amplicon-based RNAi construct targeting beta-C1 gene gives enhanced resistance against cotton leaf curl disease. 3 Biotech 11:. https://doi.org/10.1007/s13205-021-02816-6
Ding Z-H, Gao Q, Tong X, et al (2022) MAPKs trigger antiviral immunity by directly phosphorylating a rhabdovirus nucleoprotein in plants and insect vectors. Plant Cell 34:3110–3127. https://doi.org/10.1093/plcell/koac143
Marshall JM, Taylor CE (2009) Malaria Control with Transgenic Mosquitoes. PLoS Med 6:e1000020-
Acknowledgments
We acknowledge the facilities supported by DBT BUILDER (grant no. BT/INF/22/SP45382/2022) and DST FIST-II (grant no. SR/FST/LSII-046/2016(C)) to SC. SK acknowledges the University Grants Commission for a JRF fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Communicated by Robert H.A. Coutts.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Gupta, N. & Chakraborty, S. Geminiviral betasatellites: critical viral ammunition to conquer plant immunity. Arch Virol 168, 196 (2023). https://doi.org/10.1007/s00705-023-05776-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00705-023-05776-9