Abstract
Foot-and-mouth disease (FMD) is a major worldwide viral disease in animals, affecting the national and international trade of livestock and animal products and leading to high economic losses and social consequences. Effective control measures of FMD involve prevention through vaccination with inactivated vaccines. These inactivated vaccines, unfortunately, require short-term protection and cold-chain and high-containment facilities. Major advances and pursuit of hot topics in vaccinology and vectorology are ongoing, involving peptide vaccines, DNA vaccines, live vector vaccines, and novel attenuated vaccines. DIVA capability and marker vaccines are very important in differentiating infected animals from vaccinated animals. This review focuses on updating the research progress of these novel vaccines, summarizing their merits and including ideas for improvement.
Highlights
-
Shortcomings of current vaccination strategies
-
Promising novel vaccines for FMD eradication
-
Reverse genetics and new molecular technologies leading to vaccine improvement
-
Marker vaccines and FMD control policy
-
Immunoregulatory gene addition and FMD vaccine immunogenicity
Similar content being viewed by others
Introduction
Foot-and-mouth disease (FMD) is a highly contagious transboundary disease of wild and domestic cloven-hoofed animals, including cattle, swine, goats, and sheep [2]. Nasopharyngeal infection in cattle and oropharyngeal infection in swine are the starting points of foot-and-mouth disease virus (FMDV) infection, which are subsequently followed by systematic spread, with typical vesicular lesions in the mouth, interdigital cleft, coronary band, udder, teat, and claws [3, 108]. One of the dangers posed by FMDV is that animals can become carriers of the virus [98]. Carrier ruminant animals are identified based on analysis of their oro- and nasopharyngeal tissues [1, 5, 6, 109, 114, 134]. Despite the isolation of FMDV or its RNA from these animals, their role is still unclear [98]. Susceptible animals are infected with FMDV through direct or indirect contact between animals, or via fomites and airborne aerosols [13, 23]. Strict biosecurity measures, together with compulsory vaccination, have been implemented for eradicating or controlling FMD in Europe and some South American and African countries [91]. The primary obstacle to FMDV control is the epidemiological complexity in the domestic-wild animal interface, including maintenance of the virus in these animals. Infection of naïve animals can lead to grievous economic and financial losses related to elimination and control of infection, as occurred in England in 2001 [101].
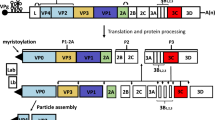
The FMDV RNA genome contains a large open reading frame (ORF) that encodes four proteins that form an icosahedral capsid without an envelope that encloses the positive-sense RNA genome. The structural proteins VP1, VP2, VP3 and VP4 are encoded by the genes 1D, 1B, 1C, and 1A, respectively, and the nonstructural proteins (NPs) are encoded by the genes 2A, 2B, 2C and 3A, 3B, 3Cpro, 3Dpol and Lpro [80]. The structural proteins are encoded within the FMD polyprotein P1 region, while the P2 and P3 regions encode the NPs responsible for FMDV maturation and replication (Fig. 1). The 5′ and 3′ untranslated regions (UTRs) are important for replication and translation of the viral genome [25]. FMDV varies antigenically and is found as seven serologically and immunologically distinct serotypes: A, O, C, SATs 1-3, and Asia-1 [118]. Variant strains within these serotypes undergo continuous antigenic and genomic evolution. Immunization with one serotype, or even a different strain of the same serotype, does not necessarily provide immunity to another serotype of another strain in the same serotype. The distribution of the different serotypes is variable and unequal in endemic regions; for instance, there are four serotypes in Africa (SAT1-3, A, O, and C), three serotypes in South America (A, O, and C), and four serotypes in Asia (Asia 1, A, O, and C) [118].
Broad species tropism, growth in international trade, a high infectivity rate, animal and human movement and activity, population growth, multiple modes of transmission, wide genetic diversity, a rapid replication rate, excretion of virus in large amounts, rapid changes in the environment, and extraordinary transmissibility make FMDV difficult and complex to harness and keep under control. All of these factors contribute to rapid re-emergence of FMD. To control the disease, several measures have to be considered, including eradication or prophylactic vaccination campaigns together with strict hygienic measures and control, including restriction policies and biosecurity measures. Various factors affect disease outcome and severity, including previous immunization or infection, species susceptibility, and the inherent viral properties of the serotype and its genetic makeup [2, 111]. The severity of FMD is high in young animals, with a higher mortality rate due to myocardium degeneration, whereas adult animals generally clear infection within two weeks [2, 4]. However, the mortality rate can sometimes be low in young animals, especially in an endemic area, due to acquired resistance through maternal antibodies.
An ideal vaccine be safe, induce a protective immune response in a single-shot vaccination, induce rapid and long-lasting immunity, have a low cost, and allow differentiation between vaccinated and infected animals (Fig. 2). Several new types of vaccines, including DNA vaccines, peptide vaccines, live-vector vaccines, and others have been developed to surmount the drawbacks of the inactivated vaccines. Each vaccine type has its own advantages and limitations. It is crucial for a vaccine to have DIVA (differentiation of infected from vaccinated animals) capability when applied for eradication strategies, and for it to induce immunity rapidly from a single inoculation in cases of emergency vaccination. Most of the new vaccines – novel attenuated, live-vector, DNA, and peptide – can be produced safely and have DIVA capability. All of them are free of risk to the vaccinated animals, except DNA vaccines, which have a low but finite probability of recombining with other genomes, and attenuated vaccines, which have the potential to revert to a virulent state. Vaccination strategies and schedules are generally dependent on the FMDV strains found in each pool. Worldwide globalization, international trade, transient populations, and mass animal movements pose a threat to regions where some imported strains are not included in the vaccine formulation.
Vaccines used to protect against FMD: update and future outlook
Inactivated vaccines
Most of the usable commercial vaccines for FMD are inactivated vaccines produced by treatment with binary ethyleneimine (BEI) to eliminate NPs. These vaccines are either monovalent, bivalent, or multivalent [40, 90]. They are oil-emulsion-, aqueous-, or aluminum-based inactivated vaccines. After concentration of the FMDV antigen, this kind of vaccine can be preserved for a long time in liquid nitrogen. The killed vaccine may be conventionally concentrated to the equivalent of three times the 50% protective dose (PD50), or additionally concentrated to the equivalent of six times the PD50, with a higher-potency effect. This highly concentrated, high-potency vaccine is mainly used as an emergency vaccine in FMD-free countries. Factors such as the antigen used, the purpose of its application, and the manufacturer are the main determinants of how much antigen is present and its concentration [21, 51]. The extremely concentrated vaccine protects against challenge within one week. Most instructions and guidelines recommend two primary injections with one month in between, followed by repeated injections every four to six months for animals up to two years old, and then additional boosters repeated yearly [90]. The major disadvantages of the presently utilized inactivated vaccines include a requirement for a highly controlled laboratory, a biosafety III facility to avoid FMDV release during vaccine production, the need to include several different serotypes, which may stress the animal’s immune system, and the need to be kept cold, as FMDV is a heat-sensitive virus [32]. Most of these vaccines, unfortunately, do not prevent primary infection and protect only from generalization, with a likelihood that more than half of the vaccinated animals will become carriers, with only DIVA assays able to distinguish vaccinated from diseased animals [32, 110]. The modern marker inactivated vaccine is a BEI-inactivated avirulent FMDV with several adjuvants. It contains intrinsic DIVA NS markers within the Lpro protein and 3AB protein [70]. The inactivated FMD vaccine is able to protect mice against challenge, and the humoral and cellular immune response is improved when vaccination is preceded by injection of a chemokine CCL20 plasmid as an adjuvant [64].
The immunogenicity of FMD vaccines inactivated using binary ethyleneimine is comparable to that of vaccines inactivated using ethyleneimine or N-acetyl ethyleneimine for inactivation. On the other hand, binary ethyleneimine in FMD vaccine preparation substantially mitigates the possible hazards related to manipulating pure ethyleneimine and other aziridines [8]. Formaldehyde inactivation can also be safe [11]. Another inactivation method, using virion-associated endonuclease, was found to be equivalent or superior to those including ethyleneimine or formaldehyde in potency tests in guinea pigs [38]. Nonchemical hydrostatic pressure (HP) inactivation could be a simple, cheap, safe, and reproducible method of viral vaccine production [60].
Live attenuated vaccines
FMDV is subject to attenuation, either via conventional means, by passing through cultured cells, or by novel means, utilizing molecular virology techniques to deoptimize or delete some genes. BHK-21 cells have been used to prepare mouse-attenuated live FMD vaccines for immunization of cattle [85]. Some modifications and cloning in BHK-21 cells were achieved after that in 1969 [86]. In one study, the live attenuated vaccine for FMD was demonstrated to protect vaccinated animals from developing lesions, with only one exception – one vaccinated animal developed a fever [79].
The new attenuated FMD vaccines are considered to be more stable than previous strains. They also have less risk of reverting to virulence than traditional ones. Detailed investigations for identifying virulence genes are crucial for developing better live attenuated vaccines. One of these virulence determinants is the viral leader protease, which inhibits induction of beta interferon mRNA and blocks the innate immunity of the host animal. Deletion of the gene for this protease has been shown to render the virus avirulent in swine and cattle. An in-frame shift in this gene also leads to its attenuation in cattle. Neither the leaderless nor the in-frame vaccine causes viremia or clinical signs after aerosol inhalation, but the leaderless variant becomes less disseminated than the in-frame variant. Leaderless mutants have also been observed to undergo partial reversion to virulence [22].
Various innovative approaches have been used to attenuate FMDV, including generation of a leaderless virus (LLV), deletion of Lpro, which produces a virus that induces a strong but inadequate protective antibody response in swine and cattle [29, 79], and excision of the conserved SAP domain from Lpro, which produces a virus that protects swine as early as two days postimmunization against homologous challenge. Higher induction of interferon-stimulated genes (ISGs) in embryonic bovine kidney cell lines affects the mRNA level of the antiviral response, and this needs more in vivo investigation in cattle [39], utilizing chimeric FMDV with bovine rhinitis B virus (BRVB) Lpro, which is closely related to the FMDV Lpro [117]. The chimeric virus is attenuated in cattle but still shows a low level of virulence in pigs and induces strong protective immunity against challenge with the homologous FMDV strain. Another strategy for attenuation of FMDV is codon pair deoptimization [38]. These novel strategies achieve virus attenuation while eliciting high neutralizing antibody titers in mice and swine.
DNA vaccines
A DNA vaccine is usually a plasmid containing the target sequence of interest (a microbial gene) under the control of a promoter for gene expression and induction of an immune response. The main features of DNA vaccines are as follows: 1) They simulate both T and B cells. 2) They are not stressful to the immune system of the vaccinated animal. 3) They are safe to use due to the lack of infectious agents. 4) They are easy to manufacture and produce. 5) They are stable and do not require a cold-chain facility. 6) They can include marker genes with DIVA capability and can be modified quickly to include field strain sequences and can contain multiple antigenic sites [68, 97]. The main challenges of DNA vaccines are that they require multiple doses with large amounts of DNA to trigger their effect. Antibodies induced by DNA vaccination have the potential to target the host DNA [92]. Another disadvantage is that they are used to produce target protein antigens, but not lipopolysaccharide antigens. After inoculation, plasmid DNA is taken up by the host cells, which express the viral proteins, which are then delivered to the ER and cleaved by cellular proteases into peptides that are later loaded onto MHC I in the ER and presented at the cell surface, leading to an immune response [18, 42].
Plasmid DNA vaccines encoding an empty capsid or containing a modified full-length FMDV genome, as well as ones expressing small regions, alone or together with immunoregulatory genes, have been used experimentally in animal models, including swine and cattle [14, 125]. The prominent shortcomings facing DNA vaccination are their requirement for large amounts of DNA, with several doses needed to achieve a protective effect. A protective response was induced in swine vaccinated with DNA encoding the FMDV capsid protein and the 3D RNA polymerase [26]. A bifunctional DNA vaccine producing antisense RNA directed to the FMDV 5′ UTR and expressing the VP1 protein, has been developed and shown to induce a rapid inhibitory effect and immune response against FMDV infection in mice [128]. Guinea pigs vaccinated with pcDNA3.1/P12X3C were fully protected against FMDV challenge. However, unsatisfactory outcomes were obtained when animals were injected with plasmid pcDNA3.1/P12X3C together with protein 3D [55]. The plasmid expressing the replicating genome pP12X3C on the other hand, provoked a stronger immune response, in swine vaccinated by the intramuscular, intradermal, or gene gun route but pWRMHX lacking a cell-binding site incompletely protected animals from challenge with highly virulent FMD [14].
In another study, DNA vaccines expressing B- and T-cell epitopes protected mice from FMDV infection despite the lack of a specific humoral response upon challenge [19]. An advance in these vaccines included expression of both B- and T-cell epitopes via some modification. Directing antigen-presenting cells (APCs) provided complete protection against challenge [20]. A DNA vaccine encoding B- and T-cell epitopes directed to class II swine leukocyte antigens provided protection to FMDV-challenged pigs [20].
Coexpression of Bcl-Xl antiapoptotic proteins with FMDV T- and B-cell epitopes led to a great improvement in the T cell response, underlining their potential in vaccine development [61].
A study using DNA nanoparticle vaccines revealed improvement in the immunological parameters and state of protection provided by pVAC FMDV VP1–OmpA in guinea pigs [87]. Calcium phosphate nanoparticles prepared with an FMDV P1-3CD gene construct protected guinea pigs and mice against viral challenge [56].
Single plasmids tend to provide stronger immunity than a combination with other plasmids. Careful evaluation before practical application is needed when using multiple plasmids [120]. Changes in ambient temperature also influence DNA vaccination in animals. Chronic heat stress (CHS) treatment has a negative impact on the immune response to FMDV DNA vaccination and significantly impairs the cellular immune response [58].
A priming immunization with a replicase-based DNA vaccine followed by a protein boost has been used in bovine calves for induction of IFN-γ [35]. This is an strategy that can clinically protect against FMDV challenge, particularly when a DNA vaccine is combined with GM-CSF and delivered by electroporation [46]. Coinjection of Isatis indigotica extract with a DNA vaccine is a beneficial way to improve DNA vaccine efficacy. Isatis indigotica extract has an adjuvant effect that enhances the immune response against viruses [28]. Coexpression with IL-2 in cis was shown to enhance the specific immune response and provide protection against homologous challenge [131]. Interleukin 15 enhanced the systemic and mucosal immunity induced by the DNA vaccine [121]. Insertion of CpG DNA into a DNA vaccine enhances the immune response against FMDV in guinea pigs [69]. MTT and 3H-thymidine incorporation assays have demonstrated good CMI responses to poly(D,L‐lactide‐co‐glycolide (PLG) microparticles of adjuvanted DNA in guinea pigs using ID-pVAC [62]. Additionally, the use of a cationic PLG microparticle to coat the DNA vaccine results in a long-term immune response against FMDV in guinea pigs [94]. An interleukin-2-enhanced immune response is elicited by a DNA vaccine when it is co-administered in swine [124]. Adjuvantation with PLG considerably boosts the efficacy of an FMD DNA vaccine [30]. A Sindbis-virus-derived plasmid (Psincp) did not improve the humoral immune response of a DNA vaccine expressing FMDV P1-2A3C3D given via intradermal injection and achieved higher humoral immunity [43]. The OmpA protein has a synergistic effect on the immunogenic FMD DNA vaccine construct when administered to guinea pigs via mannosylated chitosan nanoparticles by various routes [88]. Lactobacillus SFMD-1 has shown promise in mice as a carrier in a protective DNA vaccine against FMDV [71]. An oral DNA vaccine delivered by attenuated Salmonella choleraesuis C500, has been shown to induce cellular and humoral immunity against FMDV in rabbits [72].
Based on a large number of experimental tests, some cytokines have been identified as effective adjuvants of DNA vaccines. Interleukins have a crucial effect on DNA vaccine potency and enhance the immune response. For instance, IL-6 enhances the cell-mediated immune response and promotes maturation of dendritic cells and their immune function [112], IL-9 enhances the antigen-specific cytotoxic T lymphocyte response [137], IL-15 enhances mucosal and cellular immune responses and IFN- γ production induced by FMD DNA vaccines [121], IL-18 increases the immunogenicity of vaccines, CSF enhances the immune response [78], INF-α/β enhances the cell-mediated immune response and promotes maturation of dendritic cells and their immune function, INF-γ augments both cellular and humoral immune responses [104], and IL-1 and IL-2 promote antibody responses [102, 131].
Peptide vaccines
Peptide vaccines have many advantages over inactivated vaccines, such as relatively low-cost production, stability, and producibility on a large scale without the need for using infectious FMDV during its manufacture. Most peptide subunit vaccines are dependent on carrier proteins, such as ovalbumin or bacterial toxoid, conjugated with the peptide. These carriers must fulfil the criteria of potency and safety as well as being able to be produced easily on a large-scale with low production costs.
Peptide vaccines consist of a single linear peptide [7] corresponding to the FMDV capsid proteins or containing T-cell and/or B-cell epitopes [119]. At first, the peptides used corresponded to the C-terminal half of VP1 (residues 200-213) or to the G-H loop, which contains a B-cell epitope (residues 141-160), but these were not sufficiently protective in animal challenge experiments and induced only a limited T-cell response. A possible explanation of the limited protection and immune response is the hypervariability of the G-H loop domain [115]. Optimization of B and T sites via addition of an artificial T helper site and extensive flanking sequences resulted in some protection in pigs. Complex mixtures of peptides corresponding to several antigenic variants are more immunogenic than single peptides. A multiple-epitope recombinant vaccine provided complete protection against a challenge with the FMDV O/China/99 strain in swine, with high levels of anti-FMDV-specific antibodies at 30 days postvaccination [103]. As humoral immunity often requires conformational epitopes with the appropriate 3D structure, employing a 3D conformational peptide results in complete protection. Additionally, poly(I:C) addition is crucial for inducing interferon gamma and T cytotoxic cytokines [24]. A dendrimer strategy using one set of FMDV T-cell epitope branched out into four sets of B-cell epitope in a radially branched macromolecule shape has been shown to result in complete protection in swine and cattle [17, 34]. A commercial FMD synthetic-peptide vaccine for the prevention of pig FMD (the UBITh® vaccine) was generated by United Biomedical, Inc. (UBI) and licensed for use in Taiwan and mainland China (www.unitedbiomedical com). A multiepitope chimeric recombinant protein containing five tandem repeats of a B-cell epitope (VP1 residues 136-162) derived from different FMDV variants and one T-cell epitope (3A residues 21-35) called “5BT” has been demonstrated to elicit antibodies in mice [67].
Dendrimer peptides B4T and B2T evoked specific humoral immune responses and partially protected against the challenge with a heterologous strain in cattle [106]. B4T and B2T peptides elicited similarly robust T cell responses, and all animals showed high levels of IgG1 in the serum and mucosa; 40% of the animals in the B4T group and 20% in the B2T group produced IgA antibodies.
A conformational neutralizing epitope on the VP1 protein of type A FMDV, 135YxxPxxxxxGDLG147, has been identified [74, 105] and used for epitope-based vaccines with suitable companion MAb-based diagnostic assays [73].
Live viral vector vaccines
Delivery of immunogenic viral structural proteins can be achieved easily using viral vectors to provoke a cell-mediated and humoral immune response through their expression in vector-infected cells. These viral vectors, which act as a vehicle for the sequence of interest, include vaccinia virus, fowlpox virus, pseudorabies virus, alphaviruses, replication-defective human adenovirus virus, and Semliki Forest virus. A recombinant Sendai virus containing the P1 gene of FMDV triggered a high level of specific humoral and cellular immunity in vaccinated mice [130]. Another recombinant virus expressing FMDV epitopes that has been used as a viral vector to induce protective immunity in swine is bamboo mosaic virus [129]. Vaccination with recombinant infectious bovine rhinotracheitis virus (IBRV) expressing FMDV epitopes induced protective levels of anti-FMDV humoral antibodies in calves and protected them from challenge with virulent IBRV [66]. A bovine enterovirus expressing an FMDV epitope was also generated, but was not tested in a challenge experiment [31]. In a rabbit model, a recombinant bovine herpesvirus-1 displaying the FMDV VP1 gene induced a high level of neutralizing antibodies [95].
The recombinant PRV-FMD VP1 virus under the control of a gG promoter was not able to induce protective immunity in swine to viral challenge but was able to mitigate the clinical symptoms of infection [93]. In another study, a trivalent recombinant pseudorabies virus (PRV) against porcine parvovirus and FMDV was constructed and evaluated. It was able to protect against PRV challenge in mice, and its protective antibodies were measured by serum neutralization test (SNT) and indirect ELISA [57].
Another type of live viral vector used for protection against FMD are the adenoviruses, including canine and human adenoviruses. Expression of the FMDV VP1 protein using a canine adenovirus type 2 vector vaccine provokes a humoral response in a porcine model [75] and was also shown to protect guinea pigs in another study carried out by De Vleeschauwer et al. [36].
A recombinant adenovirus vaccine vector expressing P1 of FMDV induced partial protection against FMD in immunized cattle [99] and conferred protection against viral challenge in mice when expressing capsid proteins [135]. Swine inoculated with bivalent Ad5A24+O1 produced neutralizing antibodies (NA) against both O1 and A24, but the overall level of antibody production was substantially lower than that induced by a monovalent Ad5-A24 vaccine or a commercial FMD vaccine [126]. A single dose of Ad5-A24 provided early protection against challenge with the homologous virus [83]. Notably, monovalent live vector vaccines generally induced higher levels of humoral immunity than bivalent viral vector vaccines designed to provide protection against different FMD serotypes.
The potency of a replication-deficient Ad-FMD vector vaccine was found to be boosted by poly(ICLC), resulting in protection of challenged animals even when a low dose was used and despite the absence of measurable FMDV-specific NA at the time of challenge [37]. Interferon alpha expression by adenoviruses, together with an FMDV subunit vaccine, conferred instant and immediate protection against FMD in swine [84] as well. These results highlight the usefulness of poly ICLC and interferon alpha in enhancing the immunity provided by the Ad-FMD vector vaccine and decreasing the minimal protective dose.
The adenovirus-vectored FMDV subunit vaccine protected all vaccinated animals against FMDV dissemination [89]. The safety of replication-deficient AdtA24 vaccine was assessed in an extensive range of cattle studies, achieving safety-related specifications for U.S. regulatory requirements [9]. A recombinant Ad5-FMD was shown to be a safe, effective, and cross-reactive vaccine that is appropriate for use in outbreaks or in prevention strategies for FMDV control in swine [45].
A recombinant adenovirus expressing the whole FMDV capsid and 3C protease of serotype O bestowed protection on swine and guinea pigs [76]. Partial protection was achieved against FMDV in cattle immunized with a recombinant adenovirus vector expressing the precursor polypeptide (P1) of FMDV [100].
Cellular immunity and FMDV transgene delivery by Ad5-vectored vaccines have been improved via the inclusion of an RGD motif, but unfortunately, this did not noticeably affect vaccine effectiveness in cattle [82]. The ENABL® adjuvant reduced the protective dose of an AdtA24 vector vaccine and prevented the development of clinical FMD lesions following challenge of vaccinated steers with virulent FMDV at 7 or 14 days post-vaccination [10]. Recombinant Ad5-FMD functions better when used in a monovalent form, and its multivalent form is not promising [107]. These results highlight the effect of using appropriate adjuvants on the potency of viral vector vaccines.
Taken together, unfortunately, all of the experimental viral vector vaccines that have been developed for protecting against FMD either only partially protected cattle or swine or were not examined in their natural host [53, 78, 99, 132]. Unlike most viral vectors, one of them (replication-defective human adenovirus virus) is licensed to be used in emergency situations and has been shown to induce a full and complete immune response via its delivery of FMDV structural proteins [52, 81]. A trial was conducted recently to compare the immune response to this vector when interferon is encoded in the same vector vs. separately in another vector [65]. The best advantages associated with these adeno-vaccines are DIVA capability and the ability to induce both cellular and humoral immunity. They can be mass-produced and used economically in the veterinary sector, they do not require high biosecurity levels for production, and they are genetically stable (Fig. 3).
Virus-like particles (VLPs)
Several expression systems, including eukaryotic and prokaryotic systems, have been used for delivering virus-like particles (VLPs). VLPs include only FMDV capsid proteins and lack an infectious genome. Baculovirus/insect cell systems, bacteria, plants, and larvae have been utilized as systems for producing VLPs [54, 122, 123].
Bacterial toxin fusion proteins have been shown to induce mucosal immunity against FMDV antigens after intranasal administration to guinea pigs [27]. Transgenic tobacco expressing an FMDV epitope fused to a hepatitis B virus core particle in a complex structure has also been shown to protect mice [59]. Formation of VLPs and enhancement of the immunogenicity of a modified hepatitis B virus core particle fused to a multiepitope of FMDV has been established [127]. An MS2-mediated VLP vaccine against FMD has been shown to protect pigs, mice and guinea pigs [41]. As antigen carriers, chimeric rabbit haemorrhagic disease virus (RHDV)-VLPs have been shown to induce lymphoproliferative-specific T-cell responses in pigs and large numbers of IFN-γ-secreting cells against the 3A epitope and RHDV-VLP [33]. Transgenic alfalfa plants containing FMDV polyprotein P1 have been constructed and utilized as an experimental immunogens [44]. Purified chimeric virus particles (CVPs) constructed using tobacco necrosis virus A, produce a potent immune response against FMDV VP, when administered by the intramuscular route, and intranasal inoculation induced systemic and mucosal immunity in mice [133]. Oral administration of a T4 bacteriophage displaying FMDV capsid protein on its surface conferred 100% protection to challenge in mice [96]. Transgenic chloroplasts of a green alga have also been used as a source of a mucosal vaccine [113]. A small ubiquitin-like modifier (SUMO) fusion protein system utilizing E. coli expressing VP0, VP1, and VP3 capsid proteins protected guinea pigs, cattle, and swine from challenge [54]. Several studies, as mentioned above, have used plants, including alfalfa, tobacco, and tomato as a platform for VLP production. The use of edible plants makes vaccine delivery simple. A number of studies have shown protection in mouse models but the efficacy of these vaccines was not investigated in a natural host [122, 123].
Marker vaccine development and DIVA assays
Appropriate purification of viral antigens eliminates NPs and enables infected animals to be distinguished from vaccinated animals. Therefore, a combination of purified vaccines and tests for detecting anti-NP antibodies fundamentally provides a suitable vaccine/diagnostic marker system. Using these modern vaccines is very important when there is a need to control outbreaks and screen vaccinated animals to identify carriers [12]. The lack of protein 2C in purified FMDV vaccines provides a basis for distinguishing between convalescent and vaccinated animals [77].
Many vaccines have been used, together with companion diagnostic assays, as marker vaccines, such as chimeric FMDV vaccines [47], the partial VP1 G-H loop vaccine [48], FMD-negative marker vaccines [49], a vaccine with an exogenous FLAG epitope in RGD–4 [136], the Cav-P1/3C R° FMDV vaccine [36], the 3AB-truncated virus and its companion assay [15], two marker FMDV vaccine candidates (A24LL3DYR and A24LL3BPVKV3DYR) with Lpro and one of the 3B proteins deleted [116], an r3AB1-FMDV-NSP vaccine [63], a virus with the 3AB NSP region deleted as a companion diagnostic assay [16], and a Mab against the predominant and conserved “AEKNPLE” epitope in NSP used as a DIVA test [50].
Improving FMD vaccines and future outlook
Recent advances in reverse genetics and infectious cDNA technology have led to a revolution in the rational design of FMD vaccines (Fig. 4). Integration of current and ongoing advances in viral immunology and pathogenesis and better understanding of these processes are crucial for improving FMD vaccines. Safe and effective vaccines could be achieved by using reverse genetics and computational biology tools, methods that might lead to the development of new FMD vaccines with optimized capsid stability, antigenic matching, DIVA capability, and biosafety. Advances in genetic engineering and recombinant DNA technologies have resulted in the development of subunit vaccines. Advanced research on novel adjuvants and their incorporation into FMD vaccines could improve immunogenicity and even extend the duration of protection. Advances in the delivery of antigens directly to APCs – particularly dendritic cells (DCs) – by coupling epitopes to monoclonal antibodies or ligands specific for unique receptors expressed on the surface of APCs and active presentation of these antigens to target DCs or other APCs are ongoing.
References
Alexandersen S, Zhang Z, Donaldson AIJM (2002) Aspects of the persistence of foot-and-mouth disease virus in animals—the carrier problem. Microbes Infect 4:1099–1110
Alexandersen S, Zhang Z, Donaldson A, Garland A (2003) The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol 129:1–36
Arzt J, Pacheco J, Rodriguez L (2010) The early pathogenesis of foot-and-mouth disease in cattle after aerosol inoculation: identification of the nasopharynx as the primary site of infection. Vet Pathol 47:1048–1063
Arzt J, Baxt B, Grubman M, Jackson T, Juleff N, Rhyan J, Rieder E, Waters R, Rodriguez L (2011) The pathogenesis of foot-and-mouth disease II: viral pathways in swine, small ruminants, and wildlife; myotropism, chronic syndromes, and molecular virus–host interactions. Transbound Emerg Dis 58:305–326
Arzt J, Baxt B, Grubman M, Jackson T, Juleff N, Rhyan J, Rieder E, Waters R, Rodriguez LJT (2011) The pathogenesis of foot-and-mouth disease II: viral pathways in swine, small ruminants, and wildlife; myotropism. Chronic Syndr Mol Virus Host Interact 58:305–326
Arzt J, Belsham GJ, Lohse L, Bøtner A, Stenfeldt CJM (2018) Transmission of foot-and-mouth disease from persistently infected carrier cattle to naive cattle via transfer of oropharyngeal fluid. mSphere 3:e00365–00318
Bachrach HL, Moore DM, McKercher PD, Polatnick J (1975) Immune and antibody responses to an isolated capsid protein of foot-and-mouth disease virus. J Immunol 115:1636–1641
Bahnemann HG (1975) Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch Virol 47:47–56
Barrera J, Brake DA, Kamicker BJ, Purcell C, Kaptur R, Schieber T, Lechtenberg K, Miller TD, Ettyreddy D, Brough DE, Butman BT, Colby M, Neilan JG (2018) Safety profile of a replication-deficient human adenovirus-vectored foot-and-mouth disease virus serotype A24 subunit vaccine in cattle. Transbound Emerg Dis 65:447–455
Barrera J, Schutta C, Pisano M, Grubman MJ, Brake DA, Miller T, Kamicker BJ, Olutunmbi F, Ettyreddy D, Brough DE, Butman BT, Neilan JG (2018) Use of ENABL® adjuvant to increase the potency of an adenovirus-vectored foot-and-mouth disease virus serotype A subunit vaccine. Vaccine 36:1078–1084
Barteling SJ, Woortmeyer R (1984) Formaldehyde inactivation of foot-and-mouth disease virus. Conditions for the preparation of safe vaccine. Arch Virol 80:103–117
Barteling SJ (2002) Development and performance of inactivated vaccines against foot and mouth disease. Rev Sci Tech 21:577–588
Bates TW, Thurmond MC, Carpenter TE (2001) Direct and indirect contact rates among beef, dairy, goat, sheep, and swine herds in three California counties, with reference to control of potential foot-and-mouth disease transmission. Am J Vet Res 62:1121–1129
Beard C, Ward G, Rieder E, Chinsangaram J, Grubman M, Mason P (1999) Development of DNA vaccines for foot-and-mouth disease, evaluation of vaccines encoding replicating and non-replicating nucleic acids in swine. J Biotechnol 73:243–249
Bhatt M, Mohapatra JK, Pandey LK, Mohanty NN, Das B, Prusty BR, Pattnaik B (2018) Mutational analysis of foot and mouth disease virus nonstructural polyprotein 3AB-coding region to design a negative marker virus. Virus Res 243:36–43
Biswal JK, Subramaniam S, Ranjan R, Sharma GK, Misri J, Pattnaik B (2015) Marker vaccine potential of foot-and-mouth disease virus with large deletion in the non-structural proteins 3A and 3B. Biologicals 43:504–511
Blanco E, Guerra B, Beatriz G, Defaus S, Dekker A, Andreu D, Sobrino F (2016) Full protection of swine against foot-and-mouth disease by a bivalent B-cell epitope dendrimer peptide. Antiviral Res 129:74–80
Bolhassani A, Yazdi SR (2009) DNA immunization as an efficient strategy for vaccination. Avicenna J Med Biotechnol 1:71–88
Borrego B, Fernandez-Pacheco P, Ganges L, Domenech N, Fernandez-Borges N, Sobrino F, Rodríguez F (2006) DNA vaccines expressing B and T cell epitopes can protect mice from FMDV infection in the absence of specific humoral responses. Vaccine 24:3889–3899
Borrego B, Argilaguet JM, Pérez-Martín E, Dominguez J, Pérez-Filgueira M, Escribano JM, Sobrino F, Rodriguez F (2011) A DNA vaccine encoding foot-and-mouth disease virus B and T-cell epitopes targeted to class II swine leukocyte antigens protects pigs against viral challenge. Antiviral Res 92:359–363
Brehm K, Kumar N, Thulke H-H, Haas B (2008) High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 26:1681–1687
Brown CC, Piccone ME, Mason PW, McKenna TS, Grubman MJ (1996) Pathogenesis of wild-type and leaderless foot-and-mouth disease virus in cattle. J Virol 70:5638–5641
Burrows R, Mann J, Garland A, Greig A, Goodridge D (1981) The pathogenesis of natural and simulated natural foot-and-mouth disease infection in cattle. J Comp Pathol 91:599–609
Cao Y, Li B, Guan J, Yang J, Gan C (2013) A study on mutative scale straightness measurement based on uncertainty analysis. Measurement 46:145–153
Carrillo C, Tulman E, Delhon G, Lu Z, Carreno A, Vagnozzi A, Kutish G, Rock D (2005) Comparative genomics of foot-and-mouth disease virus. J Virol 79:6487–6504
Cedillo-Barrón L, Foster-Cuevas M, Belsham GJ, Lefèvre F, Parkhouse RME (2001) Induction of a protective response in swine vaccinated with DNA encoding foot-and-mouth disease virus empty capsid proteins and the 3D RNA polymerase. J Gen Virol 82:1713–1724
Challa S, Szczepanek SM, Rood D, Barrette RW, Silbart LK (2011) Bacterial toxin fusion proteins elicit mucosal immunity against a foot-and-mouth disease virus antigen when administered intranasally to guinea pigs. Adv Virol 2011:11
Chen L, Lin T, Zhang HX, Su YB (2005) Immune responses to foot-and-mouth disease dna vaccines can be enhanced by coinjection with the Isatis indigotica extract. Intervirology 48:207–212
Chinsangaram J, Mason PW, Grubman MJ (1998) Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine 16:1516–1522
Choudary S, Ravikumar P, Ashok Kumar C, Suryanarayana VVS, Reddy GR (2008) Enhanced immune response of DNA vaccine (VP1-pCDNA) adsorbed on cationic PLG for foot and mouth disease in guinea pigs. Virus Genes 37:81–87
Chu J-Q, Lee Y-J, Park J-N, Kim S-M, Lee K-N, Ko Y-J, Lee H-S, Cho I-S, Kim B, Park J-H (2013) Construction of a bovine enterovirus-based vector expressing a foot-and-mouth disease virus epitope. J Virol Methods 189:101–104
International Office of Epizootics. Biological Standards Commission, & International Office of Epizootics. International Committee (2008) Manual of diagnostic tests and vaccines for terrestrial animals: mammals, birds and bees, vol 2. Office international des épizooties
Crisci E, Fraile L, Moreno N, Blanco E, Cabezón R, Costa C, Mussá T, Baratelli M, Martinez-Orellana P, Ganges L, Martínez J, Bárcena J, Montoya M (2012) Chimeric calicivirus-like particles elicit specific immune responses in pigs. Vaccine 30:2427–2439
Cubillos C, Beatriz G, Jakab A, Clementi G, Borrás E, Bárcena J, Andreu D, Sobrino F, Blanco E (2008) Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J Virol 82:7223–7230
Dar PA, Suryanaryana VS, Nagarajan G, Reddy GR, Dechamma HJ, Kondabattula G (2013) DNA prime-protein boost strategy with replicase-based DNA vaccine against foot-and-mouth disease in bovine calves. Vet Microbiol 163:62–70
De Vleeschauwer AR, Zhou X, Lefebvre DJ, Garnier A, Watier F, Pignon C, Lacour SA, Zientara S, Bakkali-Kassimi L, De Clercq K, Klonjkowski B (2018) A canine adenovirus type 2 vaccine vector confers protection against foot-and-mouth disease in guinea pigs. Vaccine 36:2193–2198
Diaz-San Segundo F, Dias CC, Moraes MP, Weiss M, Perez-Martin E, Salazar AM, Grubman MJ, de los Santos T (2014) Poly ICLC increases the potency of a replication-defective human adenovirus vectored foot-and-mouth disease vaccine. Virology 468–470:283–292
Diaz-San Segundo F, Medina GN, Ramirez-Medina E, Velazquez-Salinas L, Koster M, Grubman MJ, de los Santos T (2016) Synonymous deoptimization of foot-and-mouth disease virus causes attenuation in vivo while inducing a strong neutralizing antibody response. J Virol 90:1298–1310
Díaz-San Segundo F, Weiss M, Pérez-Martín E, Dias CC, Grubman MJ, De Los Santos T (2012) Inoculation of swine with foot-and-mouth disease SAP-mutant virus induces early protection against disease. J Virol 86:1316–1327
Doel T (2003) FMD vaccines. Virus Res 91:81–99
Y-m Dong, G-g Zhang, X-j Huang, Chen L, H-t Chen (2015) Promising MS2 mediated virus-like particle vaccine against foot-and-mouth disease. Antiviral Res 117:39–43
Donnelly JJ, Wahren B, Liu MA (2005) DNA vaccines: progress and challenges. J Immunol 175:633–639
Dory D, Rémond M, Béven V, Cariolet R, Zientara S, Jestin A (2009) Foot-and-mouth disease virus neutralizing antibodies production induced by pcDNA3 and Sindbis virus based plasmid encoding FMDV P1-2A3C3D in swine. Antiviral Res 83:45–52
Dus Santos MJ, Carrillo C, Ardila F, Ríos RD, Franzone P, Piccone ME, Wigdorovitz A, Borca MV (2005) Development of transgenic alfalfa plants containing the foot and mouth disease virus structural polyprotein gene P1 and its utilization as an experimental immunogen. Vaccine 23:1838–1843
Fernandez-Sainz I, Medina GN, Ramirez-Medina E, Koster MJ, Grubman MJ, de los Santos T (2017) Adenovirus-vectored foot-and-mouth disease vaccine confers early and full protection against FMDV O1 Manisa in swine. Virology 502:123–132
Fowler V, Robinson L, Bankowski B, Cox S, Parida S, Lawlor C, Gibson D, O’Brien F, Ellefsen B, Hannaman D, Takamatsu HH, Barnett PV (2012) A DNA vaccination regime including protein boost and electroporation protects cattle against foot-and-mouth disease. Antiviral Res 94:25–34
Fowler VL, Paton DJ, Rieder E, Barnett PV (2008) Chimeric foot-and-mouth disease viruses: evaluation of their efficacy as potential marker vaccines in cattle. Vaccine 26:1982–1989
Fowler VL, Knowles NJ, Paton DJ, Barnett PV (2010) Marker vaccine potential of a foot-and-mouth disease virus with a partial VP1 G-H loop deletion. Vaccine 28:3428–3434
Fowler VL, Bashiruddin JB, Maree FF, Mutowembwa P, Bankowski B, Gibson D, Cox S, Knowles N, Barnett PV (2011) Foot-and-mouth disease marker vaccine: cattle protection with a partial VP1 G-H loop deleted virus antigen. Vaccine 29:8405–8411
Fu Y, Li P, Cao Y, Wang N, Sun P, Shi Q, Ji X, Bao H, Li D, Chen Y, Bai X, Ma X, Zhang J, Lu Z, Liu Z (2017) Development of a blocking ELISA using a monoclonal antibody to a dominant epitope in non-structural protein 3A of foot-and-mouth disease virus, as a matching test for a negative-marker vaccine. PLoS One 12:e0170560
Golde WT, Pacheco JM, Duque H, Doel T, Penfold B, Ferman GS, Gregg DR, Rodriguez LL (2005) Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: use in emergency outbreak response. Vaccine 23:5775–5782
Grubman MJ, Diaz-San Segundo F, Dias CC, Moraes MP, Perez-Martin E, De Los Santos T (2012) Use of replication-defective adenoviruses to develop vaccines and biotherapeutics against foot-and-mouth disease. Future Virol 7:767–778
Gullberg M, Lohse L, Bøtner A, McInerney GM, Burman A, Jackson T, Polacek C, Belsham GJ (2016) A prime-boost vaccination strategy in cattle to prevent foot-and-mouth disease using a “single-cycle” alphavirus vector and empty capsid particles. PLoS One 11:e0157435
Guo H-C, Sun S-Q, Jin Y, Yang S-L, Wei Y-Q, Sun D-H, Yin S-H, Ma J-W, Liu Z-X, Guo J-H (2013) Foot-and-mouth disease virus-like particles produced by a SUMO fusion protein system in Escherichia coli induce potent protective immune responses in guinea pigs, swine and cattle. Vet Res 44:48
Guo H, Liu Z, Sun S, Bao H, Chen Y, Liu X, Xie Q (2005) Immune response in guinea pigs vaccinated with DNA vaccine of foot-and-mouth disease virus O/China99. Vaccine 23:3236–3242
He Q, Mitchell A, Morcol T, Bell SJ (2002) Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin Diagn Lab Immunol 9:1021–1024
Hong Q, Qian P, Li X-M, Yu X-L, Chen H-C (2007) A recombinant pseudorabies virus co-expressing capsid proteins precursor P1-2A of FMDV and VP2 protein of porcine parvovirus: a trivalent vaccine candidate. Biotechnol Lett 29:1677–1683
Hu Y, Jin H, Du X, Xiao C, Luo D, Wang B, She R (2007) Effects of chronic heat stress on immune responses of the foot-and-mouth disease DNA vaccination. DNA Cell Biol 26:619–626
Huang Y, Liang W, Wang Y, Zhou Z, Pan A, Yang X, Huang C, Chen J, Zhang D (2005) Immunogenicity of the epitope of the foot-and-mouth disease virus fused with a hepatitis B core protein as expressed in transgenic tobacco. Viral Immunol 18:668–677
Ishimaru D, Sá-Carvalho D, Silva JL (2004) Pressure-inactivated FMDV: a potential vaccine. Vaccine 22:2334–2339
İz SG, Döşkaya M, Borrego B, Rodriguez F, Gürüz Y, Gürhan İD (2013) Co-expression of the Bcl-xL antiapoptotic protein enhances the induction of Th1-like immune responses in mice immunized with DNA vaccines encoding FMDV B and T cell epitopes. Vet Res Commun 37:187–196
Jadav SK, Reddy KS, Rashmi BR, Dechamma HJ, Ganesh K, Suryanarayana VVS, Reddy GR (2011) Improved immune response by ID-pVAC: a secretory DNA vaccine construct delivered by PLG micro particles against foot and mouth disease in guinea pigs. Res Vet Sci 91:86–89
Jaworski JP, Compaired D, Trotta M, Perez M, Trono K, Fondevila N (2011) Validation of an r3AB1-FMDV-NSP ELISA to distinguish between cattle infected and vaccinated with foot-and-mouth disease virus. J Virol Methods 178:191–200
Jayeshbhai C, Hajam IA, Verma AK, Bhanuprakash V, Kondabattula G, Kishore S (2018) Chemokine CCL20 plasmid improves protective efficacy of the Montanide ISA™ 206 adjuvanted foot-and-mouth disease vaccine in mice model. Vaccine 36:5318–5324
Kim S-M, Park J-H, Lee K-N, Kim S-K, You S-H, Kim T, Tark D, Lee H-S, Seo M-G, Kim B (2015) Robust protection against highly virulent foot-and-mouth disease virus in swine by combination treatment with recombinant adenoviruses expressing porcine alpha and gamma interferons and multiple small interfering RNAs. J Virol 89:8267–8279
Kit M, Kit S, Little SP, Di Marchi RD, Gale C (1991) Bovine herpesvirus-1 (infectious bovine rhinotracheitis virus)-based viral vector which expresses foot-and-mouth disease epitopes. Vaccine 9:564–572
Lee H-B, Piao D-C, Lee J-Y, Choi J-Y, Bok J-D, Cho C-S, Kang S-K, Choi Y-J (2017) Artificially designed recombinant protein composed of multiple epitopes of foot-and-mouth disease virus as a vaccine candidate. Microb Cell Factories 16:33
Leitner WW, Ying H, Restifo NP (1999) DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18:765–777
Li G, Li Y, Yan W, Xu Q, Wu Y, Xie Y, You Y, Zheng Z (2001) CpG DNA enhances the immune responses elicited by the DNA vaccine against foot-and-mouth disease virus in guinea pigs. Chin Sci Bull 46:1376–1379
Li P, Lu Z, Bai X, Li D, Sun P, Bao H, Fu Y, Cao Y, Chen Y, Xie B (2014) Evaluation of a 3A-truncated foot-and-mouth disease virus in pigs for its potential as a marker vaccine. Vet Res 45:51
Li Y-G, Tian F-L, Gao F-S, Tang X-S, Xia C (2007) Immune responses generated by Lactobacillus as a carrier in DNA immunization against foot-and-mouth disease virus. Vaccine 25:902–911
Liu M, Niu X, Yan J, Yan W, Zheng Z (2006) Immure response induced by oral DNA vaccination against FMDV delivered by attenuated Salmonella choleraesuis C500. Front Biol China 1:110–114
Liu W, Yang B, Wang M, Liang W, Wang H, Yang D, Ma W, Zhou G, Yu L (2017) Identification of a conserved conformational epitope in the VP2 protein of foot-and-mouth disease virus. Arch Virol 162:1877–1885
Liu W, Yang B, Wang M, Wang H, Yang D, Ma W, Zhou G, Yu L (2017) Identification of a conformational neutralizing epitope on the VP1 protein of type A foot-and-mouth disease virus. Res Vet Sci 115:374–381
Liu Y, Hu R, Zhang S, Zhang F, Li Z, Wei X, Chen L (2006) Expression of the foot-and-mouth disease virus VP1 protein using a replication-competent recombinant canine adenovirus type 2 elicits a humoral antibody response in a porcine model. Viral Immunol 19:202–209
Lu Z, Bao H, Cao Y, Sun P, Guo J, Li P, Bai X, Chen Y, Xie B, Li D, Liu Z, Xie Q (2008) Protection of guinea pigs and swine by a recombinant adenovirus expressing O serotype of foot-and-mouth disease virus whole capsid and 3C protease. Vaccine 26:G48–G53
Lubroth J, Grubman MJ, Burrage TG, Newman JFE, Brown F (1996) Absence of protein 2C from clarified foot-and-mouth disease virus vaccines provides the basis for distinguishing convalescent from vaccinated animals. Vaccine 14:419–427
Ma M, Jin N, Shen G, Zhu G, Liu HJ, Zheng M, Lu H, Huo X, Jin M, Yin G, Ma H, Li X, Ji Y, Jin K (2008) Immune responses of swine inoculated with a recombinant fowlpox virus co-expressing P12A and 3C of FMDV and swine IL-18. Vet Immunol Immunopathol 121:1–7
Mason P, Piccone M, McKenna TS-C, Chinsangaram J, Grubman M (1997) Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology 227:96–102
Mason PW, Grubman MJ, Baxt B (2003) Molecular basis of pathogenesis of FMDV. Virus Res 91:9–32
Mayr GA, Chinsangaram J, Grubman MJ (1999) Development of replication-defective adenovirus serotype 5 containing the capsid and 3C protease coding regions of foot-and-mouth disease virus as a vaccine candidate. Virology 263:496–506
Medina GN, Montiel N, Diaz-San Segundo F, Sturza D, Ramirez-Medina E, Grubman MJ, de los Santos T (2016) Evaluation of a fiber-modified adenovirus vector vaccine against foot-and-mouth disease in cattle. Clin Vaccine Immunol 23:125–136
Moraes MP, Mayr GA, Mason PW, Grubman MJ (2002) Early protection against homologous challenge after a single dose of replication-defective human adenovirus type 5 expressing capsid proteins of foot-and-mouth disease virus (FMDV) strain A24. Vaccine 20:1631–1639
Moraes MP, Chinsangaram J, Brum MC, Grubman MJ (2003) Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine 22:268–279
Mowat GN, Brooksby JB, Pay TWF (1962) Use of BHK 21 cells in the preparation of mouse attenuated live foot-and-mouth disease vaccines for the immunization of cattle. Nature 196:655
Mowat GN, Barr DA, Bennett JH (1969) The development of an attenuated foot-and-mouth disease virus vaccine by modification and cloning in tissue cultures of BHK 21 cells. Archiv für die gesamte Virusforschung 26:341–354
Nanda RK, Edao BM, Hajam IA, Sekar SC, Ganesh K, Bhanuprakash V, Kishore S (2012) An effective mannosylated chitosan nanoparticle DNA vaccine for FMD virus. Virol Sin 27:372–375
Nanda RK, Hajam IA, Edao BM, Ramya K, Rajangam M, Chandra Sekar S, Ganesh K, Bhanuprakash V, Kishore S (2014) Immunological evaluation of mannosylated chitosan nanoparticles based foot and mouth disease virus DNA vaccine, pVAC FMDV VP1–OmpA in guinea pigs. Biologicals 42:153–159
Pacheco JM, Brum MCS, Moraes MP, Golde WT, Grubman MJ (2005) Rapid protection of cattle from direct challenge with foot-and-mouth disease virus (FMDV) by a single inoculation with an adenovirus-vectored FMDV subunit vaccine. Virology 337:205–209
Parida S (2009) Vaccination against foot-and-mouth disease virus: strategies and effectiveness. Expert Rev Vaccines 8:347–365
Paton DJ, Sumption KJ, Charleston B (2009) Options for control of foot-and-mouth disease: knowledge, capability and policy. Philos Trans R Soc B Biol Sci 364:2657–2667
Pisetsky DS (1998) Antibody responses to DNA in normal immunity and aberrant immunity. Clin Diagn Lab Immunol 5:1–6
Qian P, Li X-M, Jin M-L, Peng G-Q, Chen H-C (2004) An approach to a FMD vaccine based on genetic engineered attenuated pseudorabies virus: one experiment using VP1 gene alone generates an antibody responds on FMD and pseudorabies in swine. Vaccine 22:2129–2136
Reddy KS, Rashmi BR, Dechamma HJ, Gopalakrishna S, Banumathi N, Suryanarayana VVS, Reddy GR (2012) Cationic microparticle [poly(d, l-lactide-co-glycolide)]-coated DNA vaccination induces a long-term immune response against foot and mouth disease in guinea pigs. J Gene Med 14:348–352
Ren X-G, Xue F, Zhu Y-M, Tong G-Z, Wang Y-H, Feng J-K, Shi H-F, Gao Y-R (2009) Construction of a recombinant BHV-1 expressing the VP1 gene of foot and mouth disease virus and its immunogenicity in a rabbit model. Biotechnol Lett 31:1159–1165
Ren ZJ, Tian CJ, Zhu QS, Zhao MY, Xin AG, Nie WX, Ling SR, Zhu MW, Wu JY, Lan HY, Cao YC, Bi YZ (2008) Orally delivered foot-and-mouth disease virus capsid protomer vaccine displayed on T4 bacteriophage surface: 100% protection from potency challenge in mice. Vaccine 26:1471–1481
Saade F, Petrovsky N (2012) Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 11:189–209
Salt J (1993) The carrier state in foot and mouth disease—an immunological review. Br Vet J 149:207–223
Sanz-Parra A, Jimenez-Clavero MA, Garcı́a-Briones MM, Blanco E, Sobrino F, Ley V (1999) Recombinant viruses expressing the foot-and-mouth disease virus capsid precursor polypeptide (P1) induce cellular but not humoral antiviral immunity and partial protection in pigs. Virology 259:129–134
Sanz-Parra A, Vázquez B, Sobrino F, Cox SJ, Ley V, Salt JS (1999) Evidence of partial protection against foot-and-mouth disease in cattle immunized with a recombinant adenovirus vector expressing the precursor polypeptide (P1) of foot-and-mouth disease virus capsid proteins. J Gen Virol 80:671–679
Scudamore J, Harris D (2002) Control of foot and mouth disease: lessons from the experience of the outbreak in Great Britain in 2001. Revue scientifique et technique-Office international des épizooties 21:699–707
Shao HJ, Chen L, Su YB (2005) DNA fragment encoding human IL-1beta 163-171 peptide enhances the immune responses elicited in mice by DNA vaccine against foot-and-mouth disease. Vet Res Commun 29:35–46
Shao J-J, Wong CK, Lin T, Lee SK, Cong G-Z, Sin FWY, Du J-Z, Gao S-D, Liu X-T, Cai X-P, Xie Y, Chang H-Y, Liu J-X (2011) Promising multiple-epitope recombinant vaccine against foot-and-mouth disease virus type O in swine. Clin Vaccine Immunol 18:143–149
Shi XJ, Wang B, Zhang C, Wang M (2006) Expressions of Bovine IFN-gamma and foot-and-mouth disease VP1 antigen in P. pastoris and their effects on mouse immune response to FMD antigens. Vaccine 24:82–89
Soria I, Quattrocchi V, Langellotti C, Gammella M, Digiacomo S, Garcia de la Torre B, Andreu D, Montoya M, Sobrino F, Blanco E, Zamorano P (2017) Dendrimeric peptides can confer protection against foot-and-mouth disease virus in cattle. PLoS One 12:e0185184
Soria I, Quattrocchi V, Langellotti C, Pérez-Filgueira M, Pega J, Gnazzo V, Romera S, Schammas J, Bucafusco D, Di Giacomo S, de la Torre BG, Andreu D, Sobrino F, Blanco E, Zamorano P (2018) Immune response and partial protection against heterologous foot-and-mouth disease virus induced by dendrimer peptides in cattle. J Immunol Res 2018:12
Sreenivasa BP, Mohapatra JK, Pauszek SJ, Koster M, Dhanya VC, Tamil Selvan RP, Hosamani M, Saravanan P, Basagoudanavar SH, de los Santos T, Venkataramanan R, Rodriguez LL, Grubman MJ (2017) Recombinant human adenovirus-5 expressing capsid proteins of Indian vaccine strains of foot-and-mouth disease virus elicits effective antibody response in cattle. Vet Microbiol 203:196–201
Stenfeldt C, Pacheco JM, Rodriguez LL, Arzt J (2014) Early events in the pathogenesis of foot-and-mouth disease in pigs; identification of oropharyngeal tonsils as sites of primary and sustained viral replication. PLoS One 9:e106859
Stenfeldt C, Eschbaumer M, Rekant SI, Pacheco JM, Smoliga GR, Hartwig EJ, Rodriguez LL, Arzt J (2016) The foot-and-mouth disease carrier state divergence in cattle. J Virol 90:6344–6364
Stenfeldt C, Pacheco J, Smoliga G, Bishop E, Pauszek S, Hartwig E, Rodriguez L, Arzt J (2016) Detection of foot-and-mouth disease virus RNA and capsid protein in lymphoid tissues of convalescent pigs does not indicate existence of a carrier state. Transbound Emerg Dis 63:152–164
Stenfeldt C, Segundo D-S, De Los Santos T, Rodriguez LL, Arzt J (2016) The pathogenesis of foot-and-mouth disease in pigs. Front Vet Sci 3:41
Su B, Wang J, Wang X, Jin H, Zhao G, Ding Z, Kang Y, Wang B (2008) The effects of IL-6 and TNF-alpha as molecular adjuvants on immune responses to FMDV and maturation of dendritic cells by DNA vaccination. Vaccine 26:5111–5122
Sun M, Qian K, Su N, Chang H, Liu J, Shen G (2003) Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol Lett 25:1087–1092
Sutmoller P, Gaggero AJTVR (1965) Foot-and mouth diseases carriers. Vet Rec 77:968–969
Szczepanek SM, Barrette RW, Rood D, Alejo D, Silbart LK (2012) Xenoepitope substitution avoids deceptive imprinting and broadens the immune response to foot-and-mouth disease virus. Clin Vaccine Immunol 19:461–467
Uddowla S, Hollister J, Pacheco JM, Rodriguez LL, Rieder E (2012) A safe foot-and-mouth disease vaccine platform with two negative markers for differentiating infected from vaccinated animals. J Virol 86:11675–11685
Uddowla S, Pacheco JM, Larson C, Bishop E, Rodriguez LL, Rai DK, Arzt J, Rieder E (2013) Characterization of a chimeric foot-and-mouth disease virus bearing a bovine rhinitis B virus leader proteinase. Virology 447:172–180
Upadhyaya S, Ayelet G, Paul G, King DP, Paton DJ, Mahapatra M (2014) Genetic basis of antigenic variation in foot-and-mouth disease serotype A viruses from the Middle East. Vaccine 32:631–638
Wang CY, Chang TY, Walfield AM, Ye J, Shen M, Chen SP, Li MC, Lin YL, Jong MH, Yang PC (2002) Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine 20:2603–2610
Wang K-Y, Guo Y-J, Zhang Y-L, Lv K, Sun S-H (2007) Combined DNA vaccination against three animal viruses elicits decreased immunogenicity of a single plasmid in mice. Vaccine 25:4429–4436
Wang X, Zhang X, Kang Y, Jin H, Du X, Zhao G, Yu Y, Li J, Su B, Huang C, Wang B (2008) Interleukin-15 enhance DNA vaccine elicited mucosal and systemic immunity against foot and mouth disease virus. Vaccine 26:5135–5144
Wigdorovitz A, Carrillo C, Santos MJD, Trono K, Peralta A, Gómez MC, Ríos RD, Franzone PM, Sadir AM, Escribano JM (1999) Induction of a protective antibody response to foot and mouth disease virus in mice following oral or parenteral immunization with alfalfa transgenic plants expressing the viral structural protein VP1. Virology 255:347–353
Wigdorovitz A, Filgueira DP, Robertson N, Carrillo C, Sadir A, Morris T, Borca M (1999) Protection of mice against challenge with foot and mouth disease virus (FMDV) by immunization with foliar extracts from plants infected with recombinant tobacco mosaic virus expressing the FMDV structural protein VP1. Virology 264:85–91
Wong H-T, Cheng SC-S, Sin FW-Y, Chan EW-C, Sheng Z-T, Xie Y (2002) A DNA vaccine against foot-and-mouth disease elicits an immune response in swine which is enhanced by co-administration with interleukin-2. Vaccine 20:2641–2647
Wong H, Cheng S, Chan E, Sheng Z, Yan W, Zheng Z, Xie Y (2000) Plasmids encoding foot-and-mouth disease virus VP1 epitopes elicited immune responses in mice and swine and protected swine against viral infection. Virology 278:27–35
Wu Q, Moraes MP, Grubman MJ (2003) Recombinant adenovirus co-expressing capsid proteins of two serotypes of foot-and-mouth disease virus (FMDV): in vitro characterization and induction of neutralizing antibodies against FMDV in swine. Virus Res 93:211–219
Zhang YL, Guo YJ, Wang KY, Lu K, Li K, Zhu Y, Sun SH (2007) Enhanced immunogenicity of modified hepatitis B virus core particle fused with multiepitopes of foot-and-mouth disease virus. Scand J Immunol 65:320–328
Yang B, Lan X, Li X, Yin X, Li B, Han X, Li Y, Zhang Z, Liu J (2008) A novel bi-functional DNA vaccine expressing VP1 protein and producing antisense RNA targeted to 5′UTR of foot-and-mouth disease virus can induce both rapid inhibitory effect and specific immune response in mice. Vaccine 26:5477–5483
Yang C-D, Liao J-T, Lai C-Y, Jong M-H, Liang C-M, Lin Y-L, Lin N-S, Hsu Y-H, Liang S-M (2007) Induction of protective immunity in swine by recombinant bamboo mosaic virus expressing foot-and-mouth disease virus epitopes. BMC Biotechnol 7:62
Zhang G-G, Chen X-Y, Qian P, Chen H-C, Li X-M (2016) Immunogenicity of a recombinant Sendai virus expressing the capsid precursor polypeptide of foot-and-mouth disease virus. Res Vet Sci 104:181–187
Zhang H-Y, Sun S-H, Guo Y-J, Zhu W-J, Shi K, Xu G-X, Wang J-J (2008) Optimization strategy for plasmid DNAs containing multiple-epitopes of foot-and-mouth disease virus by cis-expression with IL-2. Vaccine 26:769–777
Zhang K, Huang J, Wang Q, He Y, Xu Z, Xiang M, Wu B, Chen H (2011) Recombinant pseudorabies virus expressing P12A and 3C of FMDV can partially protect piglets against FMDV challenge. Res Vet Sci 91:90–94
Zhang Y, Li J, Pu H, Jin J, Zhang X, Chen M, Wang B, Han C, Yu J, Li D (2010) Development of Tobacco necrosis virus A as a vector for efficient and stable expression of FMDV VP1 peptides. Plant Biotechnol J 8:506–523
Zhang ZD, Kitching RP (2001) The localization of persistent foot and mouth disease virus in the epithelial cells of the soft palate and pharynx. J Comp Pathol 124:89–94
Zhou G, Wang H, Wang F, Yu L (2013) Recombinant adenovirus expressing type Asia1 foot-and-mouth disease virus capsid proteins induces protective immunity against homologous virus challenge in mice. Res Vet Sci 94:796–802
Y-y Zhu, X-q Zou, H-f Bao, Sun P, X-q Ma, Z-x Liu, H-j Fan, Q-z Zhao (2018) Insertion site of FLAG on foot-and-mouth disease virus VP1 G-H loop affects immunogenicity of FLAG. J Integr Agric 17:1655–1666
Zou Q, Wu B, He X, Zhang Y, Kang Y, Jin J, Xu H, Liu H, Wang B (2010) Increasing a robust antigen-specific cytotoxic T lymphocyte response by FMDV DNA vaccination with IL-9 expressing construct. J Biomed Biotechnol 2010:562356
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Tim Skern.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamel, M., El-Sayed, A. & Castañeda Vazquez, H. Foot-and-mouth disease vaccines: recent updates and future perspectives. Arch Virol 164, 1501–1513 (2019). https://doi.org/10.1007/s00705-019-04216-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04216-x