Abstract
Parechoviruses (PeVs) are highly prevalent viruses worldwide. Over the last decades, several studies have been published on PeV epidemiology in Europe, Asia and North America, while information on other continents is lacking. The aim of this study was to describe PeV circulation in a cohort of children in Malawi, Africa. A total of 749 stool samples obtained from Malawian children aged 6 to 60 months were tested for the presence of PeV by real-time PCR. We performed typing by phylogenetic and Basic Local Alignment Search Tool (BLAST) analysis. PeV was found in 57% of stool samples. Age was significantly associated with PeV positivity (p = 0.01). Typing by phylogenetic analysis resulted in 15 different types, while BLAST typing resulted in 14 different types and several indeterminate strains. In total, six strains showed inconsistencies in typing between the two methods. One strain, P02-4058, remained untypable by all methods, but appeared to belong to the recently reclassified PeV-A19 genotype. PeV-A1, -A2 and -A3 were the most prevalent types (26.8%, 13.8% and 9.8%, respectively). Both the prevalence and genetic diversity found in our study were remarkably high. Our data provide an important contribution to the scarce data available on PeV epidemiology in Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the genus Parechovirus (PeV) within the family Picornaviridae are small single-stranded RNA viruses. PeVs belonging to the species Parechovirus A, previously known as “Human parechovirus”, infect humans and can cause a variety of symptoms, including gastrointestinal and respiratory symptoms. Currently, 19 types of PeV-A have been distinguished, named PeV-A1-19. PeV-A3 in particular is a known cause of severe neurological disease such as meningitis and encephalitis, mainly in young children [1, 2]. For assigning new clinical strains to a type, several rules and methods have been proposed: typing based on the VP1 sequence with a 75% nucleotide (nt) sequence identity threshold [3], typing based the VP1 sequence with a 77% nt sequence identity threshold and an 87% amino acid (aa) sequence identity threshold [4], and typing based on the VP3/VP1 junction region sequence with a 82% nt and 92% aa sequence identity threshold [5]. Over the last decades, PeVs have been shown to be highly prevalent around the world. While in Europe and the USA, studies usually find a PeV prevalence (i.e. prevalence of viral RNA or infectious virus in clinical or surveillance samples) between 1 and 7% [6,7,8,9,10,11], PeV prevalence in Asia has been reported to be as high as 25% [12,13,14,15]. The most prevalent types in all of these geographical regions are PeV-A1 and -A3 [2, 7,8,9,10, 12,13,14,15]. Data on PeV circulation in Africa are scarce; only three studies have been conducted – in Kenya, Côte d’Ivoire and Ghana – finding very divergent prevalences (2%, 5.2% and 24% respectively) [16,17,18]. The aim of this study was to contribute to the little knowledge available on PeV circulation in Africa. For this, we tested samples collected in a cross-sectional study in Malawi for the presence of PeV.
Materials and methods
Patients and samples
A total of 749 fecal samples collected from Malawian children included in the SevAna study on severe anemia were included in this study [19]. The samples were collected between 2002 and 2004 from children included in one of three inclusion groups: children presenting with severe anemia (hemoglobin <5g/dl), hospital controls without severe anemia, and community controls without severe anemia. All of the included children were between 6 and 60 months of age. A questionnaire on demographic and clinical information (i.e. specific respiratory, gastrointestinal, central nervous system, and other symptoms) was completed for each participant, and clinical findings were reported. The fecal samples were stored at -80°C prior to analysis.
Nucleic acid extraction and PeV detection
Nucleic acids were extracted from all samples by the method of Boom et al. [20]. An RT-PCR was performed as described previously [21] using primers PeV F31 and PeV K30 (Table 1). Samples with a Ct value ≤40 were considered to be PeV positive. Samples with a Ct value ≤30 were included for typing.
PeV typing and phylogenetic analysis
The complete VP1 region was sequenced for typing (Cremer et al., manuscript submitted). In short, a nested PCR was conducted using primers PeV R1, F1, R2 and F2 (Table 1). The PCR products were analyzed by agarose gel electrophoresis. Positive samples with a PCR fragment size of 1000-1100 base pairs (bp) were included for sequencing. The sequencing reaction was performed using a Big Dye Terminator Kit and primers PeV F2 and PeV R2 (Table 1). Sample sequences were assembled in CodonCode Aligner (version 6.0.2) and aligned with Mafft version 7 software (https://mafft.cbrc.jp/alignment/software/) using the L-INS-i method. Maximum-likelihood (ML) phylogenetic trees including all sample strains and reference strains from the GenBank database were constructed for the VP1 sequence (nt positions 2336 to 3037 of the reference genome sequence of the Harris PeV-A1 strain [accession no. L02791]) and the VP3/VP1 junction region (nt positions 2182 to 2437) using RaxML version 8.2.12 [22] with the generalized time-reversible (GTR) nucleotide substitution model, the gamma model of rate heterogeneity, and 1000 bootstrap replicates. A Neighbor-joining (NJ) tree including the same strains was constructed for the VP1 sequence alignment using Mega7 (p-distance, 1000 bootstrap replicates) [23]. In addition, the nucleotide sequences were compared to reference strains in the GenBank database using Nucleotide Basic Local Alignment Search Tool (BLASTn) (NCBI, https://blast.ncbi.nlm.nih.gov/ accessed 1st February 2018) with standard settings for megablast (i.e., word size 28; match/mismatch scores 1, -2; linear gap costs). For strains with BLASTn genotyping results that were either unclear or conflicted with results obtained by either phylogenetic method, we searched for the translated protein sequence using tBLASTn (word size, 6; substitution matrix, BLOSUM62; gap existence cost, 11; gap extension cost, 1). No sequences of the recently reclassified PeV-A19 were available in GenBank. As a result, for all methods, typing could only be performed for PeV-A1 through -A18. The newly obtained nucleotide sequences were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases with accession numbers MH339618-MH339740.
Statistical analysis
Demographics were calculated in frequencies and percentages. For age, the median and interquartile range (IQR) (Q1-Q3) were calculated. The association between gender and PeV positivity, as well as between inclusion group and PeV positivity was determined by a chi-square test. The association between PeV positivity and age was calculated using the Mann-Whitney U test. Chi-square tests were performed for the association between PeV positivity and potential PeV symptoms (gastrointestinal, respiratory and central nervous system (CNS) symptoms and fever). All statistical analyses were performed using IBM SPSS Statistics 24.
Results
PeV prevalence in Malawian children
The baseline characteristics gender and age as well as PeV prevalence were comparable among the inclusion groups (Table 2). In total, 427 (57.0%) of the 749 samples tested positive for PeV (Table 2, Fig. 1). As data on age were lacking for nine participants, the remaining 740 participants were included for analysis on the association between age and PeV-positivity (α = 0.05). PeV positivity was significantly associated with age. PeV positive participants had a mean age of 1.8 years, and PeV-negative participants had a mean age of 2.0 years (p = 0.01). The proportion of PeV-positive participants was highest in children under 1 year of age (64.0%) and declined with increasing age to 47.2% in children aged 4 years (Fig. 2). PeV positivity was not associated with gender (p = 0.6), inclusion group (p = 0.5) or potential PeV-related symptoms (gastrointestinal [p = 0.6], respiratory [p = 0.9] or CNS symptoms [p = 0.6] or fever [p = 0.8]).
Genotype distribution
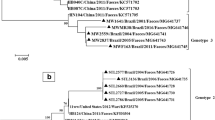
Out of 427 samples tested, 190 (44.5%) had a Ct value ≤30 and were included for additional genotyping. Good-quality sequences were obtained from 123 of 190 samples, which were typed (64.7%) (Fig. 1) using the following methods: ML phylogeny of the VP1, ML phylogeny of the VP3/VP1 junction region, NJ phylogeny of the VP1, or comparison to reference strains in GenBank using BLAST. NJ and ML phylogeny of the study strain VP1 sequences together with PeV reference strains extracted from GenBank showed that PeV-A1 was the most prevalent genotype (33/123, 26.8%), followed by PeV-A2 (17/123, 13.8%), PeV-A3 (12/123, 9.8%), and PeV-A4 (11/123, 9.0%). Other genotypes found were PeV-A5, PeV-A8 (both 10/123, 8.1%), PeV-A16 (7/123, 6.0%), PeV-A10, -A17, -A12 (4/123, 3.3%), PeV-A6, -A14 (3/123, 2.4%), PeV-A9 (2/123, 1.6%), PeV-A7 and -A11 (1/123, 0.8%) (Fig. 3, Table 3). Strain P02-4058 was distinct from all genotypes but was closest to PeV-A6, with a mean p-distance of 0.297. For PeV-A1, 28 strains grouped within the PeV-A1a cluster, while five grouped within the PeV-A1b cluster (Fig. 3).
Phylogenetic trees including our study strains and selected reference strains from the GenBank database. Phylogenetic analysis was performed on the VP1 sequence alignment (A) and VP3/VP1 junction region sequence alignment (B) using the maximum-likelihood (ML) method with the generalized time-reversible (GTR) nucleotide substitution model (1000 bootstrap replicates), and on the VP1 sequence alignment using the neighbor-joining (NJ) method (p-distance, 1000 bootstrap replicates) (C). For all study strains (P0X-XXXX, circles shaded according to their predicted genotype according to phylogenetic analyses of VP1), the year of collection is indicated (P02, 2002; P03, 2003; P04, 2004). For all reference strains (open circles), the country and year of collection and the accession number are given. Strains with inconsistent typing results between the different phylogeny methods and/or BLAST are marked in bold. The untypable strain P02-4058 is labeled as PeV-A19 in this figure, according to the typing of this strain by the Picornavirus Study Group. Bootstrap values ≥70% are shown for the branches (ML-trees) or nodes (NJ-trees)
Genotyping by ML phylogenetic analysis based on the VP1/VP3 junction region resulted in two inconsistencies compared to the analyses based on VP1: P02-4058 grouped closer to PeV-A18 (p-distance, 0.221) and -A15 (p-distance, 0.218), and P04-4393 grouped within the PeV-A8 group (Fig. 3c, Table 3). Typing the VP1 nucleotide sequences using BLAST analysis (cutoff at 77% sequence identity) resulted in inconsistencies for 11 strains compared to the phylogenetic analyses based on VP1 (P02-4058; P03-1117, -1118, -4139, -4183, -4273, -4275, -4312; P04-1310, -1475, -1556). Typing the translated aa sequences by BLAST anaysis (cutoff at 87% sequence identity) resolved the inconsistencies for six strains. The five remaining strains for which typing was not resolved included four strains that had been typed as PeV-A17 by phylogenetic analyses based on VP1 (P03-1118, P03-4312, P04-1310, P04-1556) and the untyped strain P02-4058. The PeV-A17 strains showed ≥77% nt and ≥87% aa sequence identity to both PeV-A3 and PeV-A17 strains by BLAST analysis, and therefore remained indeterminate. Strain P02-4058 showed less than 77% nt sequence identity and less than 87% aa sequence identity to any genotype and therefore remained indeterminate (Table 3 and S1).
Discussion
We found a remarkably high PeV prevalence (57%) within a cohort of Malawian children between 2002 and 2004. This prevalence is much higher than the PeV frequencies found in Asia, Europe and North America [6,7,8,9,10, 12,13,14,15, 24,25,26,27,28,29,30,31]. Recently, a PeV prevalence of 24% was found in a cohort of Ghanaian children [18], pointing towards a higher PeV prevalence in Africa than in other continents. The same seems to be the case for enteroviruses, for which the reported prevalence in this and other cohorts in Africa is higher than elsewhere in the world [32,33,34,35]. We speculate that poorer hygiene than in more developed countries is a possible explanation for the high prevalence. Alternatively, other factors may have contributed to the high prevalence of PeV found in our study. Higher prevalence of PeVs was previously reported in the rainy season compared to the dry season in Ghana [18]. The fact that the vast majority of our samples were collected during the rainy season, when malaria mainly occurs, might have contributed to the high prevalence. Furthermore, PeVs are known to circulate primarily in very young populations [1], and overall, studies that included children aged up to 5 years found higher frequencies of PeV than studies that included older children and/or adults [10, 14, 17, 18, 24, 25, 30, 31]. The fact that our population consisted solely of children between 6 and 60 months of age, will therefore have contributed to the high PeV prevalence. As there was no association between PeV positivity and inclusion groups, we consider it unlikely that hospital-acquired infections or the presence of severe anemia biased the results.
Although PeV is known to cause cases of severe disease [36, 37], PeV infection is predominantly subclinical. Seroepidemiological studies have shown that the majority of children are positive for PeV neutralizing antibodies by the age of 5 years [38,39,40,41]. In line with this, we did not find a significant association between PeV infection and clinical symptoms. PeV3 in particular is known to cause severe disease, such as meningitis, encephalitis and sepsis-like illness, mainly in children under three months of age [36, 37]. However, the 11 PeV3-positive participants in our study, all above the age of six months, did not show signs of CNS infection or sepsis.
We performed typing using different, frequently used methods: NJ phylogeny based on the VP1 sequence, ML phylogeny based on the VP1 sequence, ML phylogeny based on the VP3/VP1 junction region sequence, and typing by BLAST analysis. Typing using NJ and ML phylogenetic trees identified 15 different PeV genotypes in our study; all genotypes except PeV-A13, -A15, and -A18 were found. Typing using BLAST resulted in 14 different PeV genotypes – all genotypes except PeV-A13, -A15, -A17, and -A18– while five strains had ambiguous results and were labeled indeterminate. Strain P02-4058 remained untypable by all methods. However, a similar strain had recently been submitted to the Picornavirus Study Group and was classified as PeV-A19 after minor reorganizations of the PeV-A classification (Roland Zell, personal communication, December 2018). Strain P02-4058 was therefore typed as PeV-A19. Strain P04-4393 was typed as PeV-A14 based on the VP1-sequence and as PeV-A8 based on the VP3/VP1 junction sequence. Although recombination events in the structural parts of PeV genomes are rare, they do occur and could possibly explain the different typing results obtained by different methods [42]. Our data are in line with findings in Ghana, where all genotypes were identified except PeV-A11, -A13, -A16 and -A19 [18]. We speculate that this may reflect regional differences, with a wider variety of PeV genotypes circulating in Africa than in Europe, North America and Asia, where types other than PeV-A1-6 are rarely seen [2, 7,8,9,10, 12,13,14]. Of the genotypes found in our study, PeV-A1, -A2 and -A3 were most prevalent. While PeV-A1 is known to circulate extensively around the world, PeV-A2 is a relatively rare genotype [9, 10, 14, 27, 29]. The high prevalence of this genotype in our study is therefore notable. While PeV-A3 is also highly prevalent worldwide [9, 10, 14, 27, 29], our results are in contrast with studies conducted in Ghana and Côte d’Ivoire, where no PeV-A3 was reported [17, 18]. Since PeV-A3 circulates more widely in children under the age of 3 months [9, 26,27,28], we speculate that the proportion of PeV-A3-positive samples in this study would have been even higher if children under the age of 6 months had been included.
Different rules and methods to type PeV strains have been proposed in recent years, and currently, there is no consensus regarding which specific method to use. As a result, typing is performed using different regions and lengths of the viral genome [3,4,5] and by using different methods, such as BLAST, NJ phylogeny and ML phylogeny [13, 14, 17, 25, 27, 30, 43]. We have shown that these methods can result in different typing results for the same viral strain, leading to inconsistent and possibly incorrect typing of PeV strains. We believe that consensus on a genotyping framework, preferably based on distinct clustering in a specialized phylogenetic analysis, is needed and would provide more accurate and consistent typing in further studies.
In conclusion, we found a high frequency of PeV circulation in a population of Malawian children. We saw multiple inconsistencies in typing of strains when comparing BLAST and phylogenetic methods. However, with all methods, we found a wide variety of genotypes, with PeV-A1, -A2 and -A3 being the most prevalent types. The presence of the higher-numbered genotypes (PeV-A7-12, -A14, -A16, -A17 and -A19) and the high prevalence of PeV-A2 are especially notable. Further studies and surveillance are needed to elucidate the impact of the high prevalence and diversity of PeV and its clinical relevance on this continent. Moreover, in the future, a consensus on a typing method may be required to avoid inconsistent typing.
Abbreviations
- PeV:
-
Parechovirus
- (q)PCR:
-
(Quantitative) polymerase chain reaction
- Ct-value:
-
Threshold cycle value
- Bp:
-
Base pairs
- UTR:
-
Untranslated region
- VP1:
-
Virus protein 1
- nt:
-
Nucleotide
- aa:
-
Amino acid
- BLAST:
-
Basic Local Alignment Search Tool
References
Esposito S, Rahamat-Langendoen J, Ascolese B, Senatore L, Castellazzi L, Niesters HG (2014) Pediatric parechovirus infections. J Clin Virol 60(2):84–89. https://doi.org/10.1016/j.jcv.2014.03.003
Benschop KS, Schinkel J, Minnaar RP, Pajkrt D, Spanjerberg L, Kraakman HC, Berkhout B, Zaaijer HL, Beld MG, Wolthers KC (2006) Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis 42(2):204–210. https://doi.org/10.1086/498905
Benschop KS, Schinkel J, Luken ME, van den Broek PJ, Beersma MF, Menelik N, van Eijk HW, Zaaijer HL, VandenBroucke-Grauls CM, Beld MG, Wolthers KC (2006) Fourth human parechovirus serotype. Emerg Infect Dis 12(10):1572–1575. https://doi.org/10.3201/eid1210.051647
Nix WA, Maher K, Pallansch MA, Oberste MS (2010) Parechovirus typing in clinical specimens by nested or semi-nested PCR coupled with sequencing. J Clin Virol 48(3):202–207. https://doi.org/10.1016/j.jcv.2010.04.007
Harvala H, Robertson I, McWilliam Leitch EC, Benschop K, Wolthers KC, Templeton K, Simmonds P (2008) Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol 46(10):3446–3453. https://doi.org/10.1128/JCM.01207-08
Felsenstein S, Yang S, Eubanks N, Sobrera E, Grimm JP, Aldrovandi G (2014) Human parechovirus central nervous system infections in southern California children. Pediatr Infect Dis J 33(4):e87–e91. https://doi.org/10.1097/INF.0000000000000112
Harvala H, Calvert J, Van Nguyen D, Clasper L, Gadsby N, Molyneaux P, Templeton K, McWilliams Leitch C, Simmonds P (2014) Comparison of diagnostic clinical samples and environmental sampling for enterovirus and parechovirus surveillance in Scotland, 2010 to 2012. Euro Surveill 19(15):20772
Martin Del Valle F, Calvo C, Martinez-Rienda I, Cilla A, Romero MP, Menasalvas AI, Reis-Iglesias L, Roda D, Pena MJ, Rabella N, Portugues de la Red MDM, Megias G, Moreno-Docon A, Otero A, Cabrerizo M, Grupo de Estudio de las infecciones por enterovirus y parechovirus en n (2018) Epidemiological and clinical characteristics of infants admitted to hospital due to human parechovirus infections: a prospective study in Spain. Anales de pediatria 88(2):82–88. https://doi.org/10.1016/j.anpedi.2017.02.009
Selvarangan R, Nzabi M, Selvaraju SB, Ketter P, Carpenter C, Harrison CJ (2011) Human parechovirus 3 causing sepsis-like illness in children from midwestern United States. Pediatr Infect Dis J 30(3):238–242. https://doi.org/10.1097/INF.0b013e3181fbefc8
Sharp J, Bell J, Harrison CJ, Nix WA, Oberste MS, Selvarangan R (2012) Human parechovirus in respiratory specimens from children in Kansas City, Missouri. J Clin Microbiol 50(12):4111–4113. https://doi.org/10.1128/JCM.01680-12
van der Sanden S, de Bruin E, Vennema H, Swanink C, Koopmans M, van der Avoort H (2008) Prevalence of human parechovirus in the Netherlands in 2000 to 2007. J Clin Microbiol 46(9):2884–2889. https://doi.org/10.1128/JCM.00168-08
Chiang GPK, Chen Z, Chan MCW, Lee SHM, Kwok AK, Yeung ACM, Nelson EAS, Hon KL, Leung TF, Chan PKS (2017) Clinical features and seasonality of parechovirus infection in an Asian subtropical city, Hong Kong. PLoS One 12(9):e0184533. https://doi.org/10.1371/journal.pone.0184533
Guo Y, Duan Z, Qian Y (2013) Changes in human parechovirus profiles in hospitalized children with acute gastroenteritis after a three-year interval in Lanzhou, China. PLoS One 8(7):e68321. https://doi.org/10.1371/journal.pone.0068321
Ito M, Yamashita T, Tsuzuki H, Kabashima Y, Hasegawa A, Nagaya S, Kawaguchi M, Kobayashi S, Fujiura A, Sakae K, Minagawa H (2010) Detection of human parechoviruses from clinical stool samples in Aichi, Japan. J Clin Microbiol 48(8):2683–2688. https://doi.org/10.1128/JCM.00086-10
Patil PR, Ganorkar NN, Gopalkrishna V (2018) Epidemiology and genetic diversity of human parechoviruses circulating among children hospitalised with acute gastroenteritis in Pune, Western India: a 5-years study. Epidemiol Infect 146(1):11–18. https://doi.org/10.1017/S095026881700262X
Breiman RF, Cosmas L, Njenga M, Williamson J, Mott JA, Katz MA, Erdman DD, Schneider E, Oberste M, Neatherlin JC, Njuguna H, Ondari DM, Odero K, Okoth GO, Olack B, Wamola N, Montgomery JM, Fields BS, Feikin DR (2015) Severe acute respiratory infection in children in a densely populated urban slum in Kenya, 2007–2011. BMC Infect Dis 15:95. https://doi.org/10.1186/s12879-015-0827-x
Cristanziano VD, Bottcher S, Diedrich S, Timmen-Wego M, Knops E, Lubke N, Kaiser R, Pfister H, Kabore Y, D’Alfonso R (2015) Detection and characterization of enteroviruses and parechoviruses in healthy people living in the South of Cote d’Ivoire. J Clin Virol 71:40–43. https://doi.org/10.1016/j.jcv.2015.08.004
Graul S, Bottcher S, Eibach D, Krumkamp R, Kasmaier J, Adu-Sarkodie Y, May J, Tannich E, Panning M (2017) High diversity of human parechovirus including novel types in stool samples from Ghanaian children. J Clin Virol 96:116–119. https://doi.org/10.1016/j.jcv.2017.10.008
Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, de Haan RJ, Phiri AI, Malange P, Khoka M, Hulshof PJ, van Lieshout L, Beld MG, Teo YY, Rockett KA, Richardson A, Kwiatkowski DP, Molyneux ME, van Hensbroek MB (2008) Severe anemia in Malawian children. N Engl J Med 358(9):888–899. https://doi.org/10.1056/NEJMoa072727
Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J (1990) Rapid and simple method for purification of nucleic acids. J Clin Microbiol 28(3):495–503
Beld M, Minnaar R, Weel J, Sol C, Damen M, van der Avoort H, Wertheim-van Dillen P, van Breda A, Boom R (2004) Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control. J Clin Microbiol 42(7):3059–3064. https://doi.org/10.1128/JCM.42.7.3059-3064.2004
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874. https://doi.org/10.1093/molbev/msw054
Benschop K, Thomas X, Serpenti C, Molenkamp R, Wolthers K (2008) High prevalence of human Parechovirus (HPeV) genotypes in the Amsterdam region and identification of specific HPeV variants by direct genotyping of stool samples. J Clin Microbiol 46(12):3965–3970. https://doi.org/10.1128/JCM.01379-08
Braun LE, Renaud C, Fairchok MP, Kuypers J, Englund JA, Martin ET (2012) Human Parechovirus and other enteric viruses in childcare attendees in the era of rotavirus vaccines. J Pediatr Infect Dis Soc 1(2):136–143. https://doi.org/10.1093/jpids/pis005
Cabrerizo M, Diaz-Cerio M, Munoz-Almagro C, Rabella N, Tarrago D, Romero MP, Pena MJ, Calvo C, Rey-Cao S, Moreno-Docon A, Martinez-Rienda I, Otero A, Trallero G (2017) Molecular epidemiology of enterovirus and parechovirus infections according to patient age over a 4-year period in Spain. J Med Virol 89(3):435–442. https://doi.org/10.1002/jmv.24658
Fischer TK, Midgley S, Dalgaard C, Nielsen AY (2014) Human parechovirus infection, Denmark. Emerg Infect Dis 20(1):83–87. https://doi.org/10.3201/eid2001.130569
Ghanem-Zoubi N, Shiner M, Shulman LM, Sofer D, Wolf D, Marva E, Kra-Oz Z, Shachor-Meyouhas Y, Averbuch D, Bechor-Fellner A, Barkai G, Kinarty A, Gershstein V, Ephros M (2013) Human parechovirus type 3 central nervous system infections in Israeli infants. J Clin Virol 58(1):205–210. https://doi.org/10.1016/j.jcv.2013.06.001
Pellegrinelli L, Bubba L, Galli C, Anselmi G, Primache V, Binda S, Pariani E (2017) Epidemiology and molecular characterization of influenza viruses, human parechoviruses and enteroviruses in children up to 5 years with influenza-like illness in Northern Italy during seven consecutive winter seasons (2010–2017). J Gen Virol 98(11):2699–2711. https://doi.org/10.1099/jgv.0.000937
Sano K, Hamada H, Hirose S, Sugiura K, Harada S, Koizumi M, Hara M, Nishijima H, Taira M, Ogura A, Ogawa T, Takanashi JI (2018) Prevalence and characteristics of human parechovirus and enterovirus infection in febrile infants. Pediatr Int 60(2):142–147. https://doi.org/10.1111/ped.13467
van der Sanden SM, Koopmans MP, van der Avoort HG (2013) Detection of human enteroviruses and parechoviruses as part of the national enterovirus surveillance in the Netherlands, 1996–2011. Eur J Clin Microbiol Infect Dis 32(12):1525–1531. https://doi.org/10.1007/s10096-013-1906-9
Brouwer L, van der Sanden SMG, Calis JCJ, Bruning AHL, Wang S, Wildenbeest JG, Rebers SPH, Phiri KS, Westerhuis BM, van Hensbroek MB, Pajkrt D, Wolthers KC (2018) High frequency of polio-like Enterovirus C strains with differential clustering of CVA-13 and EV-C99 subgenotypes in a cohort of Malawian children. Arch Virol. https://doi.org/10.1007/s00705-018-3878-7
Khetsuriani N, Helfand R, Pallansch M, Kew O, Fowlkes A, Oberste MS, Tukei P, Muli J, Makokha E, Gary H (2009) Limited duration of vaccine poliovirus and other enterovirus excretion among human immunodeficiency virus infected children in Kenya. BMC Infect Dis 9:136. https://doi.org/10.1186/1471-2334-9-136
Rakoto-Andrianarivelo M, Guillot S, Iber J, Balanant J, Blondel B, Riquet F, Martin J, Kew O, Randriamanalina B, Razafinimpiasa L, Rousset D, Delpeyroux F (2007) Co-circulation and evolution of polioviruses and species C Enteroviruses in a district of Madagascar. PLoS Pathog 3(12):e191. https://doi.org/10.1371/journal.ppat.0030191
Sadeuh-Mba SA, Bessaud M, Massenet D, Joffret ML, Endegue MC, Njouom R, Reynes JM, Rousset D, Delpeyroux F (2013) High frequency and diversity of species C Enteroviruses in Cameroon and neighboring countries. J Clin Microbiol 51(3):759–770. https://doi.org/10.1128/JCM.02119-12
Khatami A, McMullan BJ, Webber M, Stewart P, Francis S, Timmers KJ, Rodas E, Druce J, Mehta B, Sloggett NA, Cumming G, Papadakis G, Kesson AM (2015) Sepsis-like disease in infants due to human parechovirus type 3 during an outbreak in Australia. Clin Infect Dis 60(2):228–236. https://doi.org/10.1093/cid/ciu784
Midgley CM, Jackson MA, Selvarangan R, Franklin P, Holzschuh EL, Lloyd J, Scaletta J, Straily A, Tubach S, Willingham A, Nix WA, Oberste MS, Harrison CJ, Hunt C, Turabelidze G, Gerber SI, Watson JT (2017) Severe Parechovirus 3 infections in young infants-Kansas and Missouri, 2014. J Pediatric Infect Dis Soc. https://doi.org/10.1093/jpids/pix010
Karelehto E, Brouwer L, Benschop K, Kok J, Basile K, McMullan B, Rawlinson W, Druce J, Nicholson S, Selvarangan R, Harrison C, Lankachandra K, Van Eijk H, Koen G, De Jong M, Pajkrt D, Wolthers K (2018) Seroepidemiology of human parechovirus 3 neutralizing antibodies among children and adults in the Netherlands, USA and Australia. Emerg Infect Dis 25(1):148–152. https://doi.org/10.3201/eid2501.180352
Tanaka S, Aoki Y, Matoba Y, Yahagi K, Itagaki T, Matsuzaki Y, Mizuta K (2016) Seroepidemiology of human parechovirus types 1, 3, and 6 in Yamagata, Japan, in 2014. Microbiol Immunol 60(12):854–858. https://doi.org/10.1111/1348-0421.12456
Watanabe K, Hirokawa C, Tazawa T (2016) Seropositivity and epidemiology of human parechovirus types 1, 3, and 6 in Japan. Epidemiol Infect. https://doi.org/10.1017/s0950268816001795
Westerhuis B, Kolehmainen P, Benschop K, Nurminen N, Koen G, Koskiniemi M, Simell O, Knip M, Hyoty H, Wolthers K, Tauriainen S (2013) Human parechovirus seroprevalence in Finland and the Netherlands. J Clin Virol 58(1):211–215. https://doi.org/10.1016/j.jcv.2013.06.036
Benschop KS, de Vries M, Minnaar RP, Stanway G, van der Hoek L, Wolthers KC, Simmonds P (2010) Comprehensive full-length sequence analyses of human parechoviruses: diversity and recombination. J Gen Virol 91(Pt 1):145–154. https://doi.org/10.1099/vir.0.014670-0
Chen H, Yao Y, Liu X, Xiao N, Xiao Y, Huang Y, Chen Q, Yu S (2014) Molecular detection of human parechovirus in children with acute gastroenteritis in Guangzhou, China. Arch Virol 159(5):971–977. https://doi.org/10.1007/s00705-013-1915-0
Funding
The SevAna study was supported by a grant (064722) from the Wellcome Trust. Our study, using the SevAna study samples, did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
KW and DP designed the study. LB, EK, AH, XT, AB, JC, MBvH, BW, DA, SK, SR and KP were involved in sample collection and/or processing. LB and EK analyzed the results. LB wrote the first version of the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
The SevAna study was ethically approved by the Ethics Committees of the College of Medicine, University of Malawi, and the Liverpool School of Tropical Medicine, United Kingdom. For our present study, no ethical approval was required.
Additional information
Handling Editor: Zhenhai Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brouwer, L., Karelehto, E., Han, A.X. et al. High frequency and diversity of parechovirus A in a cohort of Malawian children. Arch Virol 164, 799–806 (2019). https://doi.org/10.1007/s00705-018-04131-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-04131-7