Abstract
Influenza A virus (IAV) infection is a major public health threat leading to significant morbidity and mortality. The emergence of drug-resistant virus strains highlights the urgent need to develop novel antiviral drugs with alternative modes of action. Pentagalloylglucose (PGG), a naturally occurring polyphenolic compound, possesses a broad spectrum of biological activities. In this study, we found that PGG has anti-influenza-virus activity, and investigated its possible mechanism(s) of action in vitro. Both pre-incubation of virus prior to infection and post-exposure of infected cells with PGG significantly inhibited virus yields. Influenza-virus-induced hemagglutination of chicken red blood cells was inhibited by PGG treatment, suggesting that PGG can inhibit IAV infection by interacting with the viral hemagglutinin. PGG did not affect viral protein synthesis or nuclear transport of viral nucleoprotein (NP) but greatly reduced plasma membrane accumulation of NP protein at the late stage of the replication cycle. Furthermore, PGG significantly reduced virus budding and progeny virus release from infected cells. This study revealed for the first time that PGG can inhibit IAV replication with a dual mode of action and offers new insights into its underlying mechanisms of antiviral action.
Similar content being viewed by others
Introduction
Influenza A virus (IAV) is a segmented, negative-sense, single-stranded RNA virus belonging to the family Orthomyxoviridae [16]. It causes influenza, which is an acute, highly transmissible respiratory infectious disease in humans and animals. Annual seasonal epidemics and occasional pandemics of influenza result in significant morbidity and mortality in both humans and animals worldwide. Furthermore, the emergence of the highly pathogenic H5N1 avian influenza virus, which was associated with a mortality rate in excess of 60% in infected individuals [35], as well as the 2009 flu pandemic, a global outbreak of a new swine-origin strain of H1N1 influenza virus, have raised significant public-health concerns about the emergence of a potential novel highly pathogenic pandemic influenza virus strain [5, 7, 17]. Vaccination is one of the most effective means of prophylactic antiviral therapy, while antiviral medications constitute the first line of treatment following infection. Two classes of antiviral drugs, including M2 channel blockers and neuraminidase inhibitors, have proven to be clinically effective against influenza. However, due to the high mutation rate of these viruses, the emergence of drug-resistant viral strains against both classes of drugs has been reported [22, 36]. This highlights the urgent need for discovering novel antiviral drugs with alternative modes of action. Recently, accumulating evidence has shown that inhibition of intracellular signaling cascades required for virus replication is a novel alternative approach for anti-influenza therapy. The advantage of this strategy is that it can avoid the emergence of drug-resistant virus strains due to the fact that the target of the drug is a host factor that is not affected by virus mutation [19–21].
Since many traditional medicinal plants have been reported to have strong antiviral activity [6, 30, 37], they offer a rich source for discovering novel antiviral compounds. In order to explore novel active compounds against IAV, we screened a number of natural compounds purified from different Chinese medicinal plants. 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG), a naturally occurring polyphenolic compound abundant in several medicinal plants, was found to exhibit anti-influenza virus activity at non-cytotoxic concentrations. A number of in vitro and in vivo studies have previously shown that PGG exhibits a wide range of biological activities [41], including anti-inflammatory [15], antioxidant [29], anti-angiogenic [25], antitumor [13], and antibacterial activity [40], and a broad range of antiviral activity against respiratory syncytial virus (RSV) [39], hepatitis B virus (HBV) [18] and herpes simplex virus (HSV) [27, 28, 31]. Moreover, it has been shown to have an inhibitory effect on viral enzymes such as integrase and reverse transcriptase of human immunodeficiency virus (HIV-1) [1] and NS3 protease of hepatitis C virus (HCV) [8]. Although the underlying mechanisms of its antiviral action remain to be fully elucidated, the wide spectrum of its antiviral activity against different viruses suggests that PGG may target common critical steps in virus-cell interaction rather than a specific viral pathogen.
In the present study, we investigated the antiviral activity and possible mechanism(s) of action of PGG against IAV in vitro.
Materials and methods
Compound
1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG) (chemical structure shown in Fig. 1a) was isolated from the branches and leaves of Phyllanthus emblica Linn and purified as described previously [42]. The purity levels achieved were over 98%. PGG was dissolved in dimethyl sulfoxide (DMSO) and diluted with culture medium for the following experiments.
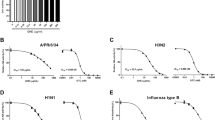
Inhibitory effects of PGG on viral yields. (a) Chemical structure of 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG). (b) Effect of PGG treatment (1.56 to 12.5 μg/ml) on virus titer. MDCK cells were infected with A/WSN/33 (MOI = 0.001) and subsequently treated with serial twofold dilutions of PGG or 0.1% (v/v) of DMSO. Culture supernatants were harvested at 24 and 48 hours postinfection (p.i.), and virus titers were determined by plaque assay. (c) Growth kinetics of A/WSN/33 virus in MDCK cells. Cells were infected with A/WSN/33 virus (MOI = 0.001) and subsequently treated with PGG (6.25 and 12.5 μg/ml) or DMSO (0.1%, v/v). Virus yields were determined at 8, 24, 36, 48 h p.i. (d) and (e) Effect of PGG on multiple replication of A/PR8/34 and A/HK/8/68 viruses in MDCK cells, respectively. Cells were infected with virus (MOI = 0.001) and subsequently treated with PGG (6.25 μg/ml, 12.5 μg/ml) or 0.1% (v/v) of DMSO. Virus yields were determined at 8 and 24 h p.i. (f) Effect of PGG on multiple replication of A/WSN/33 virus (MOI = 0.01) in A549 cells. (g) and (h) Effect of PGG on single step replication of A/PR8/34 and A/HK/8/68 viruses (MOI = 1) in A549 cells, respectively. Virus yields were determined at 8 h p.i. Values represent the mean of PFU/ml from three independent experiments, and error bars show the standard deviation of the mean. The asterisks indicate a significant difference between PGG and DMSO treatment, *P < 0.01
Cells, viruses and virus infections
Mardin-Darby canine kidney (MDCK) cells and human alveolar epithelial cell line A549 cells were cultured in minimum essential medium (MEM, Invitrogen) and Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen), respectively, supplemented with 10% (v/v) fetal bovine serum (FBS, Cell Culture Bioscience) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Influenza virus strains A/WSN/33 (H1N1), A/PR8/34 (H1N1) and A/HK/8/68 (H3N2) were propagated in 10-day-old embryonated chicken eggs. The allantoic fluids were harvested at 4 days after inoculation and stored in the freezer (−80°C). For virus infections, confluent cells were incubated with diluted virus solutions in serum-free medium for 1 hour at 37°C at the indicated multiplicities of infection (MOI). After the adsorption period, the viral inocula were removed, and cells were washed twice with PBS (-), a Ca2+/Mg2+-free-phosphate buffer solution. The cells were maintained in MEM or DMEM (supplemented with 0.5% FBS) containing 0.1% (v/v) DMSO or PGG at the indicated concentrations at 37°C in a humidified 5% CO2 incubator. 0.1% (v/v) DMSO was used as a vehicle control. For multiple replication of influenza virus strains A/PR8/34 and A/HK/8/68, the medium was additionally supplemented with 10 μg/ml trypsin (Sigma-Aldrich).
WST-1 assay
The cytotoxicity and cell-based antiviral activity of PGG were evaluated by water-soluble tetrazolium-1 (WST-1) assay [14]. For cytotoxicity analysis, confluent MDCK cells in 96-well plates (Greiner Bio-One) were incubated with serial two-fold dilutions of PGG in MEM. Antiviral activity determinations were carried out in parallel. Serial two-fold dilutions of PGG were added to cells, followed by the addition of virus inocula of 100 TCID50 (50% tissue culture infective dose) per well. After incubation for 72 hours, WST-1 (Dojindo Chemicals) solution (5 mM of WST-1 in 0.2 mM of 1-methoxy-5-methylphenaziniummethyl sulfate) was added to a final concentration of 0.25 mM. The optical density (OD) was measured 4 hours later by scanning at 450 nm and 650 nm reference wavelengths in an Emax precision microplate reader (Molecular Devices). Three independent experiments were carried out, and each experiment was performed in triplicate. The percentage of viable cells was compared with untreated controls and plotted against the concentration of the compound, and linear regression analysis was performed using Microsoft Excel software to calculate the 50% cytotoxic concentration (CC50) and the 50% effective concentration (EC50). The selectivity index (SI) for PGG was calculated by dividing the CC50 by the EC50 (CC50/EC50).
Plaque-forming unit assay (PFU assay)
The titers of infectious virus in culture supernatants harvested at the indicated time points were determined by PFU assay. Confluent MDCK cells in a 6-well plate were infected with serial 10-fold dilutions of the virus in a serum-free medium. After washing twice with PBS (-), cells were overlaid with MEM containing 0.8% (w/v) low-melting agarose, 0.1% (w/v) BSA, 1% (v/v) vitamins, and 0.03% (w/v) glutamine. In the case of A/PR8/34 and A/HK/8/68, 10 μg/ml trypsin was also added. After 3 days of incubation, cells were fixed with ethanol:acetic acid (v/v = 1:1) for 1 hour at room temperature and stained with 2.5% (w/v) Amino Black 10B after removal of the overlaying agarose gel. The plaques were counted by visual examination. Means and standard deviations were calculated from three independent experiments.
Time-of-addition assay
Time-of-addition experiments were performed in which PGG was added at different time intervals over a 24-hour incubation period. MDCK cells (6 × 105 cells/well) were seeded into 12-well plates and infected with virus at an MOI of 0.001. PGG (12.5 μg/ml) treatment or DMSO (0.1%, v/v) treatment was performed before, during, or after viral infection. At 24 hours post-infection (p.i.), culture supernatants of infected cells with different treatments were harvested, and the virus titers were determined by PFU assay. The detailed procedures for each treatment are as follows: (1) Pre-treatment of cells before virus infection: MDCK cells were pre-treated with PGG or DMSO and incubated at 37°C for 2 hours. After removal of the pre-treatment medium, the cells were washed twice with PBS (-) and infected with influenza virus. At 24 h p.i., cell supernatants were collected, and virus yields were determined by the PFU assay. (2) Pre-treatment of virus before virus infection: The virus (3 × 103 PFU) was pre-incubated with PGG or DMSO on ice for 1 hour. The mixture of virus and PGG or DMSO was then added to MDCK cells and incubated at 37°C for 1 hour. Cells were then washed twice and cultured in fresh medium for 24 hours. (3) Treatment of cells during virus infection: PGG or DMSO was administered together with the virus to the cells. After infection, cells were washed twice and cultured in fresh medium for 24 hours. (4) Treatment of cells after virus infection: After virus infection, cells were treated with PGG or DMSO for the indicated durations (as shown in Fig. 2a) and cultured in fresh medium for 24 hours.
Mode of action of PGG against influenza A virus. MDCK cells were infected with A/WSN/33 (MOI = 0.001) and treated with PGG (12.5 μg/ml) or 0.1% (v/v) DMSO (the control). The culture supernatant was collected at 24 h p.i., and virus yields were determined by plaque assay. (a) Different PGG treatment protocols. (b) PGG was added to cells before, during or after virus infection, or virus was pre-incubated with PGG prior to infection. (c) PGG was added to cells after virus infection at different time points (3, 6, 9 and 12 h p.i.). (d) Infected cells were exposed to PGG for different periods of time after infection (0-3 h, 0-6 h, 0-9 h and 0-12 h p.i.). The virus titers from the PGG-treated cells are presented as a percentage of the control (0.1% (v/v) DMSO). Values represent the mean of three independent experiments, and error bars show the standard deviation of the mean. The asterisks indicate significant differences between DMSO and PGG treatment, *P < 0.01
Western blotting
MDCK cells (2 × 105 cells/well) were seeded into 24-well plates, infected with virus (MOI = 1), and treated with PGG (12.5 μg/ml) or DMSO (0.1%, v/v). At 0, 3, 6, 9 and 12 h p.i., cells were collected and lysed in sample buffer. An aliquot of 5 μl of each lysate was subjected to SDS-PAGE using a 10% separation gel. Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore) for Western blot analysis, which was performed using mouse monoclonal antibodies against NP, HA and M1 proteins of influenza virus A (Santa Cruz Biotech) and α-tubulin (Sigma-Aldrich). Bound antibodies were visualized using an enhanced chemiluminescence (ECL) Plus Kit (GE Healthcare Life Sciences).
Indirect immunofluorescence microscopy
MDCK cells were grown on glass coverslips, infected with virus (MOI = 3), and subsequently treated with PGG (12.5 μg/ml) or DMSO (0.1%, v/v) after infection. At the indicated time points p.i., cells were fixed with 4% paraformaldehyde (PFA) in PBS for 15 minutes, permeabilized with 0.02% Triton X-100 in PBS for 15 minutes, and incubated with anti-NP monoclonal antibody (Santa Cruz Biotech) for 1 hour at 37°C. After washing with PBS, the cells were incubated with a goat anti-mouse IgG H- and L-chain-specific biotin conjugate (Calbiochem) for 1 hour at 37°C. Then, streptavidin fluorochrome conjugate (Calbiochem) was added to the cells and incubated at 37°C for 1 hour. Cell nuclei were counterstained with Hoechst 33342 (Sigma-Aldrich) for 10 minutes at room temperature. Slides were examined under a fluorescence microscope (Carl Zeiss) using a 100× Plan Apo objective, and the images were captured using the AxioVision software platform.
Transmission electron microscopy (TEM)
MDCK cells were infected with virus (MOI = 3), treated with PGG (12.5 μg/ml) or DMSO (0.1%, v/v) for 12 hours, fixed in 3% glutaraldehyde (pH 7.2) for 1.5 hours, and post-fixed in 1% osmium tetroxide for 1 hour. After dehydration, cells were embedded in Spurr (Sigma-Aldrich). Several consecutive ultrathin sections were cut on an LKB Nova ultramicrotome (LKB) and then stained with saturated uranyl acetate and lead citrate. These sections were examined under a transmission electron microscope, JEM1400 (JEOL).
Quantitative real-time RT-PCR
MDCK cells (2 × 105 cells/well) were seeded into 24-well plates and then infected with virus at different MOIs (1 or 0.01), followed by treatment with PGG (12.5 μg/ml) or DMSO (0.1%, v/v) for 12 hours. The culture supernatants were collected from infected cells, and after removal of cellular debris by centrifugation, total RNA was isolated using PureLink™ Viral RNA/DNA Kits (Invitrogen) and reverse transcribed in the presence of random hexamers using a ReverTra Ace qPCR RT Kit (Toyobo). The viral genomic segment 7 of influenza virus strain A/WSN/33 was specifically amplified by polymerase chain reaction (PCR) using specific primers (sense: TCTGATCCTCTCGTCATTGCAGCAA; antisense: AATGACCATCGTCAACATCCACAGC). The cDNA was amplified by PCR using SYBR Green Realtime PCR Master Mix (Toyobo) as described by the manufacturer, using an ABI PRISM 7000 Sequence Detection System. The PCR conditions were as follows: denaturation at 95°C for 1 minute, followed by 40 cycles of 95°C for 15 seconds/60°C for 1 minute. Melting curve analysis was performed to verify the specificity of the products. A standard curve (R 2 > 0.99 within the range of 101-108 copies per reaction) was used to convert the respective cycle threshold (Ct) values to the number of viral genome copies. This standard consisted of a pCAGGS-WSN-M plasmid construct in which was cloned the full sequence of influenza virus A/WSN/33 segment 7. All samples were run in triplicate.
Hemagglutination inhibition (HI) assay
The HI assay was carried out as described by Ehrhardt et al. [9]. Briefly, serial twofold dilutions of PGG (25 μl) were prepared, mixed with an equal volume of influenza virus suspension (22 HA units/25 μl). After incubation for 1 h at 4°C, 1% (v/v) chicken erythrocytes (50 μl) in PBS (-) were added, and the sample was incubated for 30 min at room temperature.
Statistical analysis
Results were expressed as mean ± S.E.M. for three independent experiments. Student’s unpaired t-test was used to evaluate the difference between the test samples and untreated controls. A P value of <0.01 was considered statistically significant.
Results
Antiviral activity and cytotoxicity of PGG
For initial analysis of antiviral activity, PGG was tested on MDCK cells in a cell-based screening assay. The cytotoxicity of PGG was also evaluated. The EC50 value was 29.59 ± 4.32 μg/ml (31.48 ± 4.60 μM). No significant cytotoxicity was observed at concentrations of PGG up to 12.5 μg/ml. PGG showed potent inhibitory activity against influenza virus strain A/WSN/33(H1N1): the EC50 value was 2.36 ± 0.29 μg/ml (2.51 ± 0.31 μM). The selectivity index (SI), which is expressed as the ratio of CC50/EC50, was 12.54.
Inhibitory effects of PGG on virus yield
To confirm the inhibitory effects of PGG on virus replication, virus yield was investigated in MDCK cells and A549 cells after infection with different strains of influenza A virus in the presence or absence of PGG. As shown in Fig. 1b, PGG significantly inhibited influenza A/WSN/33(H1N1) virus yields from MDCK cells at 24 and 48 h p.i. in a dose-dependent manner. Maximum reduction (over 4 log10 PFU/ml) was observed at a concentration of 12.5 μg/ml. Comparison with the viral growth kinetics of PGG-treated cells and the DMSO-treated cells demonstrated that the inhibitory activity of PGG on virus yields remained stable during a 48-h period p.i. (Fig. 1c). In addition to influenza virus strain A/WSN/33(H1N1), PGG also inhibited the multiple replication of influenza virus strains A/PR8/34 (H1N1) and A/HK/8/68 (H3N2) in MDCK cells (Fig. 1d and e). Similar results were also obtained using A549 cells instead of MDCK cells. The virus yield of A/WSN/33(H1N1) from A549 cells was inhibited under conditions of multiple infection in the presence of 12.5 μg/ml PGG (Fig. 1f). A greater than 80% of inhibition in virus yield was observed when single infection was carried out with A/PR8/34 (H1N1) or A/HK/8/68 (H3N2) (Fig. 1g and h). These data indicate that PGG inhibits influenza A virus replication.
Mode of action of PGG against influenza A virus
To investigate the mode of action of PGG, time-of-addition experiments were performed (Fig. 2a). Pre-incubation of cells with PGG prior to infection showed no significant inhibitory effect on virus yield; however, a significant reduction (over 90%) in virus yield was observed when virus was pre-incubated with PGG prior to infection or cells were treated with PGG during or after infection (Fig. 2b), suggesting that PGG may have virucidal activity. In order to determine whether PGG inhibited virus yield during a specific period in the virus replication cycle, the effect on compound addition at different time intervals using MDCK cells was studied. As shown in Fig. 2c, compared to DMSO treatment, even when PGG was added 12 hours after infection, a reduction of more than 95% in virus yield was still achieved during 24 hours of infection. Furthermore, to avoid an exposure of newly formed virions to PGG prior to titration, infected cells were exposed to PGG only within a single replication cycle (0-12 hours p.i.), and then the supernatants containing PGG or DMSO were replaced with fresh medium. As shown in Fig. 2d, in comparison to DMSO treatment, PGG treatment do not affect virus yield during the first 3 hours of treatment (0-3 hours); however, a significant reduction in virus yield was observed at 0-6 hours. Similarly, at 0-9 hours and 0-12 hours, the level of inhibition reached 86% and 94%, respectively (Fig. 2d). These results suggest that PGG may interfere predominantly with the late stage of the virus replication cycle, independent of its viricidal activity.
Inhibitory effects of PGG on hemagglutination
Since influenza A viruses are able to agglutinate chicken red blood cells (RBCs) by binding of their viral envelope spike protein hemagglutinin (HA) to the receptors on RBCs, to further confirm the effect of PGG on virus adsorption to cells, a hemagglutination inhibition assay was carried out. As shown in Table 1, PGG inhibited HA activity for all the three virus strains in a concentration-dependent manner. These results suggested that PGG is capable of directly interacting with the viral glycoprotein HAs to block virus adsorption to cells.
Effects of PGG on viral protein synthesis
To determine whether the inhibitory effects of PGG treatment on viral replication were related to the production of viral proteins, the expression of viral proteins in infected cells that were treated with PGG for 3, 6, 9 or 12 hours was analyzed by Western blotting. As shown in Fig. 3, in which the viral protein levels were normalized using α-tubulin, PGG did not significantly affect the expressions of viral HA, NP and M1 proteins.
Effects of PGG on viral protein synthesis. MDCK cells were infected with A/WSN/33 (MOI = 1) and subsequently treated with 12.5 μg/ml PGG or 0.1% (v/v) DMSO after infection. At 0, 3, 6, 9 and 12 hours p.i., cells were analyzed and subjected to Western blot analysis using a monoclonal antibody against influenza A virus HA, NP and M1 proteins and anti-α-tubulin antibody (a). Protein band densities were quantified using Image J software. The relative levels of virus HA (b), NP (c) and M1 (d) protein expression were calculated by normalizing to that of α-tubulin
Effects of PGG on distribution of viral nucleoprotein (NP) in MDCK cells
To evaluate the effect of PGG on intracellular trafficking of the viral ribonucleoprotein (NP), indirect immunofluorescence staining was performed using anti-NP antibody at 3, 6, 9, and 12 h p.i. As shown in Fig. 4, viral NP accumulated in the nucleus of infected cells as early as 3 h p.i. (Fig. 4a and c), and translocation to the cytoplasm was completed within 9 h p.i. (Fig. 4i and k). No difference in the pattern of distribution of NP was observed between PGG-treated and untreated cells until 6 h p.i. The viral NP accumulated at the leading edge of cells without PGG treatment (Fig. 4i and m, arrow), but not in PGG-treated cells at 9 and 12 h p.i. (Fig. 4k and o). These results indicate that PGG did not affect nuclear entry or extranuclear translocation of NP at the early and middle stages of the replication cycle (0 to 6 hours) but interfered with the accumulation of NP on the surface of the cell membrane at the late stage of the replication cycle (9 to 12 hours).
Effects of PGG on subcellular distribution of viral nucleoprotein. MDCK cells were infected with A/WSN/33 (MOI = 3) and subsequently treated with 12.5 μg/ml PGG or 0.1% (v/v) DMSO after infection. Cells were fixed and stained for immunofluorescence at 3 hours (a to d), 6 hours (e to h), 9 hours (i to l), and 12 hours (m to p) and analyzed by fluorescence microscopy. Cells were co-stained with anti-NP antibody (a, c, e, g, i, k, m, o), and Hoechst 33342 (b, d, f, h, j, l, n, p). The arrowheads in i and m show the distribution of viral NP on the leading edge of cells
Ultrastructural analysis of virus budding by TEM
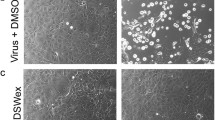
Ultrastructural analysis of cells by TEM showed that most of the mock-infected cells were smooth-surfaced or had sparsely scattered microvillar membrane protrusions (Fig. 5a and d). In contrast, many membrane protrusions were observed on the surface of infected cells that had not been treated with PGG (Fig. 5b and e), in which there were numerous budding viral particles (arrows) on the surface of the microvillar protrusions (Fig. 5g). PGG treatment reduced the appearance of microvillar protrusions on the surface of cells (Fig. 5c) and decreased the number of budding virus particles (arrows), for which virus buds are seen lining the surface of the cell membrane (Fig. 5f). Virus buds exhibited a spherical shape rather than an elongated or filamentous form (Fig. 5h). These results revealed that PGG possibly affects the surface structure of the plasma membrane, which may cause the reduction in virus assembly and budding on the surface of infected cells.
Ultrastructure of virus budding on the cell surface. MDCK cells were infected with influenza A/WSN/33 virus (MOI = 3) and subsequently treated with 12.5 μg/ml PGG or 0.1% (v/v) DMSO for 12 hours. Cells were examined by TEM as described in Materials and methods. The figure depicts mock-treated cells (a, d), infected cells treated with DMSO (b, e, g), and infected cells treated with PGG (c, f, h). Boxed areas with a dotted line are shown at a higher magnification in d, e and f. g and h represent enlarged images of the boxed areas with solid lines in e and f, respectively. The arrowheads illustrate where virions are seen pinching off from the surface of the membrane
Effects of PGG on virus particle release
To determine whether the release of total virus particles from infected cells was suppressed by PGG, we treated the infected cells with PGG within the first replication cycle (for 5 or 8 h upon infection) and determined the virus titers at 12 h postinfection. As shown in Fig. 6 a and b, in comparison to DMSO treatment, treatment with PGG for 5 or 8 h upon infection significantly inhibited the virus titer (over 90% reduction). We further analyzed the copy number of viral genomic RNA in the supernatants from infected cells at 12 h p.i. using quantitative real-time RT-PCR. A reduction of more than 70% in the amount of viral genomic RNA was observed in the culture supernatants in the presence of PGG (Fig. 6c). These results indicate that PGG significantly suppresses total virus particle release in a single replication cycle.
Effects of PGG on viral particle release. (a) and (b) Inhibitory effects of PGG on virus titer. MDCK cells were infected with A/WSN/33 (MOI = 0.01 and MOI = 1) and treated with 12.5 μg/ml PGG or 0.1% (v/v) DMSO for 5 or 8 h upon infection. The virus titer in the culture supernatant was determined at 12 h postinfection. (c) Effect of PGG on the amount of viral RNA present in the culture supernatant. MDCK cells were infected with A/WSN/33 (MOI = 0.01 and MOI = 1) and treated with 12.5 μg/ml PGG or 0.1% (v/v) DMSO for 12 hours. Viral genomic RNA (segment 7) in the culture supernatant was analyzed using quantitative real-time RT-PCR. Viral RNAs in cell culture supernatants from PGG-treated cells were compared to the control (treatment with DMSO). Values represent the mean of three independent experiments, and error bars show the standard deviation of the mean. The asterisks indicate significant differences between DMSO and PGG treatment, *P < 0.01
Discussion
In the present study, our results indicate that PGG isolated from Phyllanthus emblica Linn effectively inhibits influenza A virus replication via two mechanisms: prevention of virus adsorption and suppression of virus release. Pre-treatment of virus before infection or treatment of cells during infection greatly reduced virus yields during 24 hours of infection; however, pre-treatment of cells prior to infection did not significantly reduced virus yield. Also, treatment with PGG during the first three hours after infection did not affect virus yield. Therefore, it is conceivable that the inhibitory effect of PGG was mainly caused by the direct interaction of PGG with the virus. Numerous studies have demonstrated that plant polyphenols, including the tea catechins (-) epigallocatechin gallate (EGCG) and theaflavin digallate [34], resveratrol (RV) [26], a polyphenol-rich extract (CYSTUS052) [10], pomegranate polyphenol extract (PPE) [12], oligonol [11], and hydroxytyrosol (HT) [38], have potent antiviral activity against influenza virus that is related to the nature of their interactions with viral particles. Additionally, indirect effects of some polyphenols, such as EGCG and strictinin, on host cells that might interfere with virus-cell membrane fusion have also been suggested [23, 32]. In this study, our results demonstrated that PGG inhibits virus-induced hemagglutination of chicken red blood cells, suggesting that PGG can interact with virus particles. Analysis of the detailed mechanisms of PGG acting on viral HA is currently underway.
Notably, our results also showed that PGG can reduce virus yields at the late stage of the replication cycle, independent of its virucidal activity. This is supported by the finding that virus release is significantly reduced by PGG treatment in a single virus replication cycle. Reduced release of virus particles was evident in the results of four independent assays: (i) lower virus titers as determined by PFU assay, (ii) reduced membrane accumulation of NP protein as determined by immunofluorescence staining, (iii) decreased numbers of virus particles on the surface of the plasma membrane as determined by TEM observation, and (iv) reduced viral genomic RNA in culture supernatants as determined by quantitative real-time RT-PCR. Treatment with PGG for 5 or 8 h upon infection significantly inhibited virus release, suggesting that PGG may interfere with the steps before virus release in a late stage of the replication cycle. PGG treatment did not affect the expression of viral proteins in infected cells or the nuclear transport of the viral NP protein, but the accumulation of NP on the plasma membrane was significantly suppressed in the presence of PGG, which is in accordance with the decrease in the number of virus buds on the plasma membrane in the presence of PGG in the TEM study. In addition, PGG treatment induced a decrease in the number of microvillus-like membrane protrusions, which is the site for virus assembly and budding. It is likely that PGG acts on the cellular membrane and therefore interferes with virus budding and release.
Influenza virus budding and release are essential for the transmission of the virus and for the pathogenesis of disease. A better understanding of these processes will help us in identifying new targets for prevention of influenza virus infection. Disruption of actin microfilaments by inhibitors alters the distribution of NP at the apical plasma membrane [33]. Influenza virus NP protein is known to associate with the actin cytoskeleton, which may provide the pushing force for incorporating the vRNP complex into the bud [2]. vRNPs can also be directed to the apical budding site via their association with lipid rafts [4]. Although the molecular mechanism of transport of vRNPs to the apical plasma membrane remains to be fully elucidated, recently, the Rab11-mediated membrane trafficking pathway has been reported to be required for IAV budding [3]. We have recently reported that PGG can downregulate cofilin1, a key regulator of actin cytoskeleton dynamics, which might be associated with its anti-HSV-1 activity [28]. Whether these cellular factors, as common targets, were affected by PGG treatment needs be examined further. Moreover, release of virus particles from the surface of the plasma membrane in the late stage of influenza virus replication requires the envelope spike glycoprotein neuraminidase (NA), which has sialidase activity [24]. Whether the inhibition of virus release by PGG treatment is associated with the effect of PGG on NA activity is also an intriguing subject.
In conclusion, this study, for the first time, demonstrates that PGG possesses antiviral activity against influenza A virus in vitro. PGG inhibits productive replication of IAV not only by inhibiting virus infection but also by interfering with virus budding and release. The dual mode of action of PGG on virus observed in this study implies that PGG is a promising antiviral agent against influenza A virus.
References
Ahn MJ, Kim CY, Lee JS, Kim TG, Kim SH, Lee CK, Lee BB, Shin CG, Huh H, Kim J (2002) Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med 68:457–459
Avalos RT, Yu Z, Nayak DP (1997) Association of influenza virus NP and M1 proteins with cellular cytoskeletal elements in influenza virus-infected cells. J Virol 71:2947–2958
Bruce EA, Digard P, Stuart AD (2010) The Rab11 pathway is required for influenza a virus budding and filament formation. J Virol 84:5848–5859
Carrasco M, Amorim MJ, Digard P (2004) Lipid raft-dependent targeting of the influenza A virus nucleoprotein to the apical plasma membrane. Traffic 5:979–992
CDC (2009) Update: novel influenza A (H1N1) virus infections—worldwide, May 6, 2009. MMWR Morb Mortal Wkly Rep 58:453–458
Chuanasa T, Phromjai J, Lipipun V, Likhitwitayawuid K, Suzuki M, Pramyothin P, Hattori M, Shiraki K (2008) Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antiviral Res 80:62–70
Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM (2009) Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360:2605–2615
Duan D, Li Z, Luo H, Zhang W, Chen L, Xu X (2004) Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett 14:6041–6044
Ehrhardt C, Hrincius ER, Korte V, Mazur I, Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O, Ludwig S (2007) A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res 76:38–47
Ehrhardt C, Hrincius ER, Korte V, Mazur I, Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O, Ludwig S (2007) A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res 76:38–47
Gangehei L, Ali M, Zhang W, Chen Z, Wakame K, Haidari M (2010) Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine 17:1047–1056
Haidari M, Ali M, Ward Casscells S, 3rd Madjid M (2009) Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 16:1127–1136
Huh JE, Lee EO, Kim MS, Kang KS, Kim CH, Cha BC, Surh YJ, Kim SH (2005) Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis 26:1436–1445
Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K (1996) A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull 19:1518–1520
Kang DG, Moon MK, Choi DH, Lee JK, Kwon TO, Lee HS (2005) Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) via a nitric oxide-cGMP pathway. Eur J Pharmacol 524:111–119
Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA (2007) Fields virology. Lippincott Williams and Wilkins, New York, pp 1647–1689
Korteweg C, Gu J (2010) Pandemic influenza A (H1N1) virus infection and avian influenza A (H5N1) virus infection: a comparative analysis. Biochem Cell Biol 88:575–587
Lee SJ, Lee HK, Jung MK, Mar W (2006) In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose against hepatitis B virus. Biol Pharm Bull 29:2131–2134
Lu X, Masic A, Li Y, Shin Y, Liu Q, Zhou Y (2010) The PI3K/Akt pathway inhibits influenza A virus-induced Bax-mediated apoptosis by negatively regulating the JNK pathway via ASK1. J Gen Virol 91:1439–1449
Ludwig S, Wolff T, Ehrhardt C, Wurzer WJ, Reinhardt J, Planz O, Pleschka S (2004) MEK inhibition impairs influenza B virus propagation without emergence of resistant variants. FEBS Lett 561:37–43
Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, Wolff T, Planz O, Ludwig S (2007) Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol 9:1683–1694
Moscona A (2008) Medical management of influenza infection. Annu Rev Med 59:397–413
Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T (1993) Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res 21:289–299
Nayak DP, Balogun RA, Yamada H, Zhou ZH, Barman S (2009) Influenza virus morphogenesis and budding. Virus Res 143:147–161
Oh GS, Pae HO, Choi BM, Lee HS, Kim IK, Yun YG, Kim JD, Chung HT (2004) Penta-O-galloyl-beta-D-glucose inhibits phorbol myristate acetate-induced interleukin-8 [correction of interleukin-8] gene expression in human monocytic U937 cells through its inactivation of nuclear factor-kappaB. Int Immunopharmacol 4:377–386
Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F, Ciriolo MR, Garaci E (2005) Inhibition of influenza A virus replication by resveratrol. J Infect Dis 191:1719–1729
Pei Y, Chen ZP, Ju HQ, Komatsu M, Ji YH, Liu G, Guo CW, Zhang YJ, Yang CR, Wang YF, Kitazato K (2011) Autophagy is involved in anti-viral activity of pentagalloylglucose (PGG) against Herpes simplex virus type 1 infection in vitro. Biochem Biophys Res Commun 405:186–191
Pei Y, Xiang YF, Chen JN, Lu CH, Hao J, Du Q, Lai CC, Qu C, Li S, Ju HQ, Ren Z, Liu QY, Xiong S, Qian CW, Zeng FL, Zhang PZ, Yang CR, Zhang YJ, Xu J, Kitazato K, Wang YF (2011) Pentagalloylglucose downregulates cofilin1 and inhibits HSV-1 infection. Antiviral Res 89:98–108
Piao X, Piao XL, Kim HY, Cho EJ (2008) Antioxidative activity of geranium (Pelargonium inquinans Ait) and its active component, 1,2,3,4,6-penta-O-galloyl-beta-D-glucose. Phytother Res 22:534–538
Pleschka S, Stein M, Schoop R, Hudson JB (2009) Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol J 6:197
Quideau S, Varadinova T, Karagiozova D, Jourdes M, Pardon P, Baudry C, Genova P, Diakov T, Petrova R (2004) Main structural and stereochemical aspects of the antiherpetic activity of nonahydroxyterphenoyl-containing C-glycosidic ellagitannins. Chem Biodivers 1:247–258
Saha RK, Takahashi T, Kurebayashi Y, Fukushima K, Minami A, Kinbara N, Ichitani M, Sagesaka YM, Suzuki T (2010) Antiviral effect of strictinin on influenza virus replication. Antiviral Res 88:10–18
Simpson-Holley M, Ellis D, Fisher D, Elton D, McCauley J, Digard P (2002) A functional link between the actin cytoskeleton and lipid rafts during budding of filamentous influenza virions. Virology 301:212–225
Song JM, Lee KH, Seong BL (2005) Antiviral effect of catechins in green tea on influenza virus. Antiviral Res 68:66–74
Uyeki TM (2009) Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin Infect Dis 49:279–290
Vicente D, Cilla G, Montes M, Mendiola J, Perez-Trallero E (2009) Rapid spread of drug-resistant influenza A viruses in the Basque Country, northern Spain, 2000-1 to 2008-9. Euro Surveill 14(20):pii=19215
Wang X, Jia W, Zhao A (2006) Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res 20:335–341
Yamada K, Ogawa H, Hara A, Yoshida Y, Yonezawa Y, Karibe K, Nghia VB, Yoshimura H, Yamamoto Y, Yamada M, Nakamura K, Imai K (2009) Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antiviral Res 83:35–44
Yeo SJ, Yun YJ, Lyu MA, Woo SY, Woo ER, Kim SJ, Lee HJ, Park HK, Kook YH (2002) Respiratory syncytial virus infection induces matrix metalloproteinase-9 expression in epithelial cells. Arch Virol 147:229–242
Zhang F, Luo SY, Ye YB, Zhao WH, Sun XG, Wang ZQ, Li R, Sun YH, Tian WX, Zhang YX (2008) The antibacterial efficacy of an aceraceous plant [Shantung maple (Acer truncatum Bunge)] may be related to inhibition of bacterial beta-oxoacyl-acyl carrier protein reductase (FabG). Biotechnol Appl Biochem 51:73–78
Zhang J, Li L, Kim SH, Hagerman AE, Lu J (2009) Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res 26:2066–2080
Zhang Y-J, Nagao T, Tanaka T, Yang C-R, Okabe H, Kouno I (2004) Antiproliferative activity of the main constituents from Phyllanthus emblica. Biol Pharm Bull 27(2):251–255
Acknowledgments
This work was supported by a grant-in-aid from the Tokyo Biochemical Research Foundation and was partially supported by the Joint Funds of National Science Foundation of China (U0632010), the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences (P2008-KF07, P2008-ZZ08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, G., Xiong, S., Xiang, YF. et al. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch Virol 156, 1359–1369 (2011). https://doi.org/10.1007/s00705-011-0989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-0989-9