Abstract
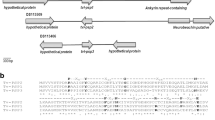
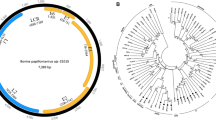
A type III Trichomonas vaginalis virus, which may be involved in transcriptional regulation of the major surface protein gene P270 of the protozoan pathogen Trichomonas vaginalis, was purified and characterized in the present study. The complete 4844-base-pair complementary DNA sequence of the viral genome reveals overlapping cap and pol genes with a putative ribosomal frame-shifting signal within the overlap region. The type III virus is related more closely to the type II virus than to the type I virus in the sequence of its ribosomal frameshift signal and in its capsid protein. Phylogenetic analysis revealed that these viruses could be grouped in the same clade as a genus distantly related to other genera in the family Totiviridae. Virus-induced P270 gene expression was only evident in Trichomonas vaginalis cells infected with either a type II or type III virus, but not with a type I virus. These findings suggest that transcription of the P270 gene is likely regulated by viral factors common to type II and type III viruses and thus provides important information for future investigation of virus-host interactions.








Similar content being viewed by others
References
Alderete JF, Kasmala L, Metcalfe E, Garza GE (1986) Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun 53:285–293
Alderete JF (1999) The Trichomonas vaginalis phenotypically varying P270 immunogen is highly conserved except for numbers of repeated elements. Microb Pathog 27:93–104
Bessarab IN, Liu HW, Ip CF, Tai JH (2000) The complete cDNA sequence of a type II Trichomonas vaginalis virus. Virology 267:350–359
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12:387–395
Esteban R, Fujimura T, Wickner RB (1989) Internal and terminal cis-acting sites are necessary for in vitro replication of the L-A double-stranded RNA virus of yeast. EMBO J 8:947–954
Fichorova RN (2009) Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol 83:185–189
Gesteland RF, Atkins JF (1996) Recoding: Dynamic reprogramming of translation. Annu Rev Biochem 65:741–768
Ghabrial SA, Nibert ML (2009) Victorivirus, a new genus of fungal viruses in the family Totiviridae. Arch Virol 154:373–379
Giedroc DP, Cornish PV (2009) Frameshifting RNA pseudoknots: Structure and mechanism. Virus Res 139:193–208
Graves A, Gardner WA (1993) Pathogenicity of Trichomonas vaginalis. Clin Obstet Gynecol 36:145–152
Heine P, McGregor JA (1993) Trichomonas vaginalis: a reemerging pathogen. Clin Obstet Gynecol 36:137–144
Honigberg BM (1978) Trichomonads of importance in human medicine, p275–754. In: Kreier JP (ed) Parasitic protozoa, vol 2. Academic Press, Inc, New York
Hsu HM, Ong SJ, Lee MC, Tai JH (2009) Transcriptional regulation of an iron-inducible gene by differential and alternate promoter entries of multiple Myb proteins in the protozoan parasite Trichomonas vaginalis. Eukaryot Cell 8:362–372
ICTVdB Descriptions (2006) 00.075.0.02. Giardiavirus In ICTVdB-The Universal Virus Database, version 4, http://www.ictvdb.org/ICTVdB/
Keister DB (1983) Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg 77:487–488
Khoshnan A, Alderete JF (1993) Multiple double-stranded RNA segments are associated with virus particles infecting Trichomonas vaginalis. J Virol 67:6950–6955
Khoshnan A, Alderete JF (1994) Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J Virol 68:4035–4038
Khoshnan A, Alderete JF (1995) Characterization of double-stranded RNA satellites associated with the Trichomonas vaginalis virus. J Virol 69:6892–6897
Kim JW, Chung PR, Hwang MK, Choi EY (2007) Double-stranded RNA virus in Korean isolate IH-2 of Trichomonas vaginalis. Korean J Parasitol 45:87–94
Liu HW, Chu YD, Tai JH (1998) Characterization of Trichomonas vaginalis virus proteins in the pathogenic protozoan T. vaginalis. Arch Virol 143:963–970
Meade JC, Shah PH, Lushbaugh WB (1997) Trichomonas vaginalis: Analysis of codon usage. Exp Parasitol 87:73–74
Meysick K, Garber GE (1995) Trichomonas vaginalis. Curr Opin Infect Dis 8:22–25
Ong SJ, Hsu HM, Liu HW, Chu CH, Tai JH (2006) Multifarious transcriptional regulation of adhesion protein gene ap65-1 by a novel Myb1 protein in the protozoan parasite Trichomonas vaginalis. Eukaryot Cell 5:391–399
Ong SJ, Hsu HM, Liu HW, Chu CH, Tai JH (2007) Activation of multifarious transcription of an adhesion protein ap65-1 gene by a novel Myb2 protein in the protozoan parasite Trichomonas vaginalis. J Biol Chem 282:6716–6725
Park Y, James D, Punja ZK (2005) Co-infection by two distinct totivirus-like double-stranded RNA elements in Chalara elegans (Thielaviopsis basicola). Virus Res 109:71–85
Petrin D, Delgaty K, Bhatt R, Garber G (1998) Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev 11:300–317
Ribas JC, Wickner RB (1996) Saccharomyces cerevisiae L-BC double-stranded RNA virus replicase recognizes the L-A positive-strand RNA 3′ end. J Virol 70:292–297
Sambrook J, Fritsch EF, Maniatis T (1989) “Molecular Cloning, A Laboratory Manual”, 2nd ed edn. Cold Spring Harbor Laboratory press, Cold Spring Harbor, New York
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Su HM, Tai JH (1996) Genomic organization and sequence conservation in type I Trichomonas vaginalis viruses. Virology 222:470–473
Tai JH, Su HM, Tsai J, Shaio MF, Wang CC (1993) The divergence of Trichomonas vaginalis virus RNAs among various isolates of Trichomonas vaginalis. Exp Parasitol 76:278–286
Tai JH, Ip CF (1995) The cDNA sequence of Trichomonas vaginalis virus-T1 double-stranded RNA. Virology 206:773–776
Tai JH, Chang SC, Ip CF, Ong SJ (1995) Identification of a satellite double-stranded RNA in the parasitic protozoan Trichomonas vaginalis infected with T. vaginalis virus T1. Virology 208:189–196
ten Dam EB, Pleij CWA, Bosch L (1990) RNA pseudoknots: translational frameshifting and readthrough on viral RNAs. Virus Genes 4:121–136
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsai CD, Liu HW, Tai JH (2002) Characterization of an iron-responsive promoter in the protozoan pathogen Trichomonas vaginalis. J Biol Chem 277:5153–5162
Wang A, Wang CC, Alderete JF (1987) Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med 166:142–150
Wang A, Yang HM, Shen KA, Wang CC (1993) Giardiavirus double-stranded RNA genome encodes a capsid polypeptide and a gag-pol-like fusion protein by a translation frameshift. Proc Natl Acad Sci USA 90:8595–8599
Yao W, Adelman K, Bruenn JA (1997) In vitro selection of packaging sites in a double-stranded RNA virus. J Virol 71:2157–2162
Yu DC, Wang CC (1996) Identification of cis-acting signals in the giardiavirus (GLV) genome required for expression of firefly luciferase in Giardia lamblia. RNA 2:824–834
Zhao Y, Zhang X, Li J, Liu Q, Yin J, Gong P (2006) Trichomonas vaginalis virus 1 isolate Changchun capsid protein gene, complete cds; and RNA-dependent RNA polymerase gene, partial cds. GenBank, Accession No. DQ528812
Acknowledgements
This work was supported by National Science Council (Grant No. NSC97-2320-B-001-005-MY3), and Academia Sincia, Taiwan, Republic of China. Electron microscopy was performed with the help of Hsiu-Jung Ma of the EM core facility at the Institute of Biomedical Sciences. We thank Dr. J.F. Alderete (School of Molecular Biosciences, Washington State University) for providing monoclonal antibody C20A3. We also appreciate the help of Mr. Douglas Platt in reading and correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The sequence data from this manuscript have been deposited with GenBank Data Libraries under accession no. AF325840.
Rights and permissions
About this article
Cite this article
Bessarab, I.N., Nakajima, R., Liu, HW. et al. Identification and characterization of a type III Trichomonas vaginalis virus in the protozoan pathogen Trichomonas vaginalis . Arch Virol 156, 285–294 (2011). https://doi.org/10.1007/s00705-010-0858-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-010-0858-y