Abstract
The worldwide prevalence of Parkinson’s disease (PD) has been constantly increasing in the last decades. With rising life expectancy, a longer disease duration in PD patients is observed, further increasing the need and socioeconomic importance of adequate PD treatment. Today, PD is exclusively treated symptomatically, mainly by dopaminergic stimulation, while efforts to modify disease progression could not yet be translated to the clinics. New formulations of approved drugs and treatment options of motor fluctuations in advanced stages accompanied by telehealth monitoring have improved PD patients care. In addition, continuous improvement in the understanding of PD disease mechanisms resulted in the identification of new pharmacological targets. Applying novel trial designs, targeting of pre-symptomatic disease stages, and the acknowledgment of PD heterogeneity raise hopes to overcome past failures in the development of drugs for disease modification. In this review, we address these recent developments and venture a glimpse into the future of PD therapy in the years to come.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A change in the therapeutic landscape has been achieved for many chronic or previously incurable diseases over the last decade. Antisense oligonucleotide or gene replacement therapy for patients with spinal muscular atrophy (Weiß et al. 2022) and the recent approval of aducanumab and lecanemab for patients with Alzheimer’s disease (Rabinovici 2021; van Dyck et al. 2023) are only selected examples for recent breakthrough developments. In the case of Parkinson’s disease (PD), many disease-modifying therapies (DMTs) have advanced from preclinical into clinical testing, but none of these approaches has yet been able to demonstrate a disease-modifying effect in clinical trials. However, new formulations of established dopaminergic drugs, device-aided applications, and new drug targets for the treatment of motor fluctuations in advanced stages have further improved the care of patients with PD. With nearly 150 registered clinical trials for disease-modifying and symptomatic therapies in the ClinicalTrials.gov database in 2022 (McFarthing et al. 2022), recent advances in biomarker development (Espay et al. 2017b), and increasing implementation of digital tools in the healthcare of PD patients (Hassan et al. 2020), the field of future PD therapies is as vibrant as ever.
In this narrative review, we cover advances in the treatment of motor symptoms in early and later stages of the disease and non-motor symptoms categorized by symptom groups, then discuss recent approaches of disease modification. Thereafter, we address the implementation of biomarker-based or genetic stratification of the PD population and sensor-based clinical monitoring into future treatment routine for PD patients. We emphasize the role of biomarkers in premotor diagnosis and target engagement during clinical trials investigating disease modification. We cover the existing evidence through author knowledge and selective PubMed searches (last search performed January 10, 2023) using the search terms: “biomarker”, “disease modification”, “early diagnosis”, “treatment monitoring”, “motor symptoms”, “non-motor symptoms”, “neurodegeneration”, “Parkinson’s disease”, “precision medicine”, “stratification”, “symptomatic treatment”, “synucleinopathies”, “telehealth”, “wearables”.
Symptomatic therapy
Early PD
In current practice, initial PD-specific drug treatment focuses on motor symptoms, mostly by oral administration. Available options include levodopa and dopa decarboxylase inhibitors, non-ergot dopamine agonists, inhibitors of the monoamine oxidase B or, less frequently, amantadine or anticholinergics. Treatment is then tailored to the severity of the symptoms, age, and pre-existing conditions of the patient (Fox et al. 2018). Response to initial therapy is usually satisfactory; nevertheless, further efforts are being made to optimize treatment in early PD stages. Tavapadon, a novel D1/D5 dopamine receptor partial agonist, is currently investigated in several phase 3 studies (NCT04201093, NCT04223193, NCT04542499, NCT04760769) to evaluate effects on motor functions in early and later stage patients with and without motor fluctuations (Table 1). Two earlier phase 2 studies for this compound had been terminated by the sponsor due to insufficient efficacy in advanced stages of PD, but the results still indicated a significant improvement in motor functions in early PD, while being generally well tolerated by patients (Riesenberg et al. 2020). Opicapone, an inhibitor of catechol-O-methyltransferase, already approved by EMA and FDA, is currently investigated in a phase 3 study to further assess possible benefits for patients with early PD without motor fluctuations (NCT04978597). A low-dose combination of two approved compounds, pramipexole and rasagiline (P2B001), is evaluated in patients with early PD. A phase 2 trial indicated a good clinical efficacy without an increased rate of adverse events (Olanow et al. 2017). The results of a phase 3 study are pending (NCT03329508).
Advanced PD
Advanced-stage PD is characterized by insufficient motor control including motor fluctuations, such as dyskinesia and OFF periods, despite optimized oral dopaminergic medication (Soileau et al. 2022). In everyday clinical routine, the 1-2-5 criteria can be used for the identification of advanced PD patients, referring to the presence of at least 1 h of troublesome dyskinesia, 2 h of OFF periods, and the intake of at least 5 doses of oral levodopa (Antonini et al. 2018b). Mechanistically, variable dopamine plasma levels due to pulsatile oral medication, erratic gastric emptying, and decreasing neuronal buffer capacity can result in motor fluctuations (Antonini et al. 2018a; Dijk et al. 2020). Therefore, symptomatic treatment in the advanced stage needs regular adaptation and new symptomatic options are needed to further improve control of motor fluctuations and increasingly debilitating non-motor symptoms.
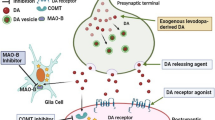
As OFF states can occur unheralded and independent from medication intake, on-demand treatment of OFF states is a growing field including the application of new formulations of well-known drugs. Orally inhaled levodopa (CVT-301) recently received marketing authorization by FDA and by EMA as a “rescue agent” for the treatment of patients during recurring OFF states. In addition, a novel sublingual film formulation of apomorphine was recently approved by the FDA for on-demand treatment in OFF state. Clinical studies reported an overall reduction of OFF state and a higher likelihood of achieving ON state for these two formulations, while being generally well tolerated, with cough, upper respiratory infections, and dyskinesia as main side effects of CVT-301 (LeWitt et al. 2019; Farbman et al. 2020) and oropharyngeal side effects, transient nausea, somnolence and dizziness in sublingual apomorphine (Olanow et al. 2020). Oral levodopa in combination with a decarboxylase inhibitor is mainly prescribed in immediate-release (IR) and controlled-release (CR) formulations. Currently, novel extended-release formulations (ER), designed to dissolve at different rates, are investigated. A recently completed phase 3 study with IPX-203 showed a significant, albeit small, reduction of OFF state in treated patients (Hauser et al. 2022). A similar oral formulation, IPX-066, had already received approval by FDA in 2015 after proving to reduce duration of OFF state and the occurrence of dyskinesia in PD patients with motor fluctuations, while being generally well tolerated (Hauser et al. 2013). However, marketing authorization for the EU was withdrawn at the request of the marketing authorization holder in 2019. WD-1603, another oral ER formulation of levodopa/carbidopa, is currently under investigation in a phase 2 study (NCT05036473). Already marketed in Japan, zonisamide has been discussed as suitable add-on therapy especially for patients in advances stages. Zonisamide is a reversible MAO-B inhibitor and T-type calcium-channel antagonist. In RCTs conducted in the Japanese population, it reduced OFF-time without increasing troublesome dyskinesia and a positive effect on tremor has been postulated (Li et al. 2020). Currently, there are no ongoing clinical trials to evaluate efficacy in other populations.
An antagonist of the dopamine D3 receptor, mesdopetam, is currently being evaluated for its effects on dyskinesia in a phase 2b study (NCT04435431). Previous studies in rodent models and a phase 1 study indicated a possible anti-dyskinetic and antipsychotic effect without deterioration of motor functions due to its physicochemical properties similar to a dopamine receptor agonist (Waters et al. 2020; Sjöberg et al. 2021). Targeting serotonergic terminals has been proposed as promising target for the treatment of dyskinesia (Politis et al. 2014; Di Luca et al. 2022), therefore buspirone/zolmitriptan (JM-010), agonists of 5-HT1A and 5-HT1B/D, respectively (NCT04377945, NCT03956979), and NLX-112, a 5-HT1A receptor agonist (NCT05148884), are currently investigated. Other oral approaches include AV-101/L-4-chlorokynurenine with antagonistic effects on the NMDA receptor (NCT04147949), CPL500036, a PDE10A inhibitor (NCT05297201), and dipraglurant, a negative allosteric modulator of glutamate receptor type 5 (mGluR5; NCT04857359).
Advanced PD leads to an increased impairment of motor and cognitive functions associated with a greater incidence of falls, which may result in injuries, reduced mobility, quality of life, and life expectancy (Fasano et al. 2017). A recent Cochrane review including 156 randomized controlled trials (RCTs) with 7939 participants showed a small to large effect of different forms of physical exercise and interventions on motor function and quality of life in PD patients, i.e., aqua-based, endurance, gait/balance, functional, and multi-domain training (Ernst et al. 2023). As of January 6th, 2023, 95 active or recruiting trials are listed in the clinicaltrials.gov registry to assess the effect of different forms of physical activity or support by assistance devices. Currently investigated pharmacologic approaches for the prevention of falls in PD patients include transdermal rivastigmine, a cholinergic drug (phase 3: NCT04226248), oral TAK-071, an allosteric modulator of the muscarinic M1 receptor (phase 2: NCT04334317), and pirepemat, modulating cortical catecholaminergic levels (phase 2: NCT05258071; Rein-Hedin et al. 2021).
For oral treatment of moderate to severe tremor resistant to usual PD medication, suvecaltamide, a T-type calcium-channel modulator, is currently evaluated in a recently initiated phase 2 study (NCT05642442).
Three well-established device-aided therapies have been used in advanced PD since the 1990s and 2000s: deep brain stimulation (DBS), Levodopa–carbidopa intestinal gel (LCIG), and continuous subcutaneous apomorphine infusion (CSAI). The latter two avoid fluctuating plasma drug levels by continuously and directly delivering dopaminergic medication into the small intestine (LCIG) or subcutaneously (CSAI) (Dijk et al. 2020), while DBS directly influences locomotor circuits of the basal ganglia (Okun 2012). Randomized trials have proven safety and significant efficacy on motor fluctuations of these device-aided therapies (Deuschl et al. 2006; Katzenschlager et al. 2018; Olanow et al. 2014; Schuepbach et al. 2013; Williams et al. 2010). Recently, jejunal application of levodopa/carbidopa and entacapone (LECIG) in a 4:1:4 ratio via a portable pump could achieve similar levodopa plasma levels with an intestinal levodopa dosage reduction of 35% compared to LCIG (Senek et al. 2020, 2017). An ongoing observational study (NCT05043103) will provide further long-term outcomes of LECIG in the next years. A main drawback of approved device-aided therapies lies in their invasiveness. In an explorative setting (NCT04778176), oral levodopa/carbidopa delivered continuously via a tooth-attached pump system (DopaFuse) reduced fluctuations of plasma levodopa levels compared to standard levodopa/carbidopa, improved ON-time without severe dyskinesia and OFF-time, while being well tolerated (SynAgile Corporation 2022). In addition, two new subcutaneous application approaches avoid invasive surgery and are potentially reversible (Rosebraugh et al. 2021a). A novel levodopa/carbidopa formulation (ND0612) with optimized aqueous solubility and stability can be delivered via a portable pump system (LeWitt et al. 2022; Olanow et al. 2021). In a 8:1 levodopa/carbidopa ratio, several phase 1 and 2 trials proved stable, dosage-proportional and thereby steerable levodopa plasma levels (LeWitt et al. 2022; Giladi et al. 2021), resulting in significant decreases in OFF-time and ON-time with troublesome dyskinesia, while increasing ON-time without troublesome dyskinesia (NCT02577523; Olanow et al. 2021). Due to the relatively large infusion volume, infusion site reactions (mostly mild and reversible nodules, erythema, hematoma, infection in up to 95% of all patients) are the most common side effects (Olanow et al. 2021; Giladi et al. 2021), resulting in a relevant dropout rate in the 1-year interim analysis of the phase 2 open-label study (NCT02726386; Poewe et al. 2021). A multicenter, double-dummy-controlled phase 3 trial of ND0612 (NCT04006210) is ongoing. In parallel, subcutaneous foslevodopa/foscarbidopa (phosphorylated levodopa/carbidopa prodrugs, ABBV-951) has been evaluated in a multicenter phase 3 trial (NCT04380142; Soileau et al. 2022) and showed significant improvement of motor fluctuations in advanced PD: the mean reduction in OFF-time (1.79 h) and the mean increase in ON-time without troublesome dyskinesia (1.75 h), each compared to oral levodopa/carbidopa alone, were equivalent to the clinical benefits documented for LCIG (respective values 1.91 h and 1.86 h; Olanow et al. 2014). Foslevodopa/foscarbidopa attained high chemical stability and > tenfold aqueous solubility compared to levodopa/carbidopa, thereby avoiding large infusion volumes and enabling high plasma levels of levodopa/carbidopa after subcutaneous application (Soileau et al. 2022; Rosebraugh et al. 2021a, 2021b). Similar to ND0612, mostly mild infusion site reactions were the predominant side effect. Additional phase 3 trials (e.g., NCT04750226, NCT03781167) will provide more safety and efficacy data of foslevodopa/foscarbidopa.
A promising novel approach for advanced and/or tremor-dominant PD patients is MR-guided focused ultrasound (MRgFUS). MRgFUS combines focused high-intensity ultrasound, administered via multiple stereotactic transducers on the skull, with MR thermography and allows non-invasive thermocoagulative target lesioning with submillimeter precision and without classical risks of open surgery (Moosa et al. 2019; Martínez-Fernández et al. 2021; Xu et al. 2021). Unilateral thalamotomy (Nucleus ventralis intermedius thalami; VIM) is the most common MRgFUS target for tremor-dominant, medication-refractory PD. A randomized, sham-controlled, prospective trial (NCT01772693) showed 62% tremor reduction on the contralateral hemibody (Bond et al. 2017). Since 2017, unilateral VIM-thalamotomy is an FDA-approved technique in the United States. The main drawback is the insufficient control of ipsilateral tremor and other PD cardinal symptoms, mainly bradykinesia and rigidity (Martínez-Fernández et al. 2021). Recently, a pilot study showed a maintenance of stable levodopa dosage and sufficient tremor control by unilateral thalamotomy for at least 6 months in early-stage tremor-dominant PD patients, thereby potentially enlarging the target group for thalamotomy (Golfrè Andreasi et al. 2022). Less evidence exists for other targets in MRgFUS. Unilateral MRgFUS of the subthalamic nucleus (STN; subthalamotomy) lead to significant reduction of PD cardinal symptoms in a randomized, sham-controlled, double-blind trial of markedly asymmetric PD patients (NCT03454425) (Martínez-Fernández et al. 2020) and bilateral subthalamotomy is currently investigated in a feasibility study (NCT03964272). Focused ablation of the globus pallidus internus (GPI; pallidotomy) reduced dyskinesia in a pilot study (NCT02263885, Eisenberg et al. 2020) and improved motor function while reducing dyskinesia in patients with motor fluctuations (NCT03319485; Krishna et al. 2023). MRgFUS pallidothalamic tractotomy reduces pallidal overinhibition without ablation of the thalamus, showed promising results in case series (Gallay et al. 2019), and is currently investigated in two open-label trials (NCT04728295, NCT04996992). Mostly transient side effects of MRgFUS comprise paraesthesia, gait disturbance, hemiparesis and—in case of subthalamotomy and pallidotomy—hemichorea, speech and visual deficits (Moosa et al. 2019; Martínez-Fernández et al. 2021). Randomized controlled trials are needed to provide further evidence for clinical outcomes of MRgFUS in PD, including bilateral application.
Non-motor symptoms
Non-motor symptoms can occur in all stages of PD, have a negative impact on the quality of life, and are associated with poor long-term outcomes (Weintraub et al. 2022). They include neuropsychiatric conditions, autonomic dysfunctions, disorders of sleep, and pain. Non-motor symptoms, especially neurocognitive symptoms, depression and pain, impact quality of life more heavily than motor symptoms (Tarolli et al. 2020). Pharmacological therapies of motor symptoms sometimes present themselves with beneficial effects on non-motor symptoms, therefore sparing adjunctive therapies and reducing polypharmacy. However, non-motor symptoms show a distinct interdependence (Marinus et al. 2018) and therapeutic effects on individual symptoms often overlap with effects on other symptoms of this spectrum. A good example is safinamide. Safinamide, a selective, reversible MAO-B inhibitor that also reduces glutamate release by blocking voltage-dependent sodium channels and modulating calcium channels, was approved in 2015 as add-on therapy to levodopa in advanced PD patients with motor fluctuations. There is evidence to suggest that safinamide also has beneficial effects on sleep, fatigue, mood, and pain (Stocchi et al. 2022) and it is currently investigated in advanced PD patients for its effect on sleep quality (NCT03968744).
Depression and anxiety affect approximately 45% of PD patients, sometimes preceding motor symptoms (Lemke et al. 2004). Treatment includes non-pharmacologic measures, i.e., cognitive behavioral therapy, physical exercise, and first-line pharmacological use of mainly selective serotonin reuptake inhibitors (SSRI), serotonin norepinephrine reuptake inhibitors (SNRI), and tricyclic antidepressants (TCA) with mixed evidence of efficacy in previous RCTs (Bomasang-Layno et al. 2015; Weintraub et al. 2022). TCAs display a disfavored safety profile with special concern for PD patients, because orthostatic hypotension, constipation, confusion, and delirium, particularly in demented patients, are known side effects (Starkstein and Brockman 2017). Non-pharmacological interventions, such as cognitive behavioral therapy and multimodal interventions, e.g., cognitive-based mindfulness therapy, showed promising and robust effects (Starkstein and Brockman 2017). Substances currently evaluated in clinical trials include a comparison of nortriptyline and escitalopram, a TCA and a SSRI (phase 3: NCT03652870), vortioxetine, a modulator of serotonin receptors and transport (phase 4: NCT04301492), psilocybin, a psychoactive alkaloid (phase 2: NCT04932434), and intravenous ketamine (phase 2: NCT04944017).
Psychosis affects up to 60% of PD patients at some point in the course of the disease (Weintraub et al. 2022). Clozapine and quetiapine (off-label) are currently the main drug treatments of PD psychosis due to their low risk of worsening extrapyramidal symptoms (Seppi et al. 2019). Still, medication with atypical antipsychotics may lead to an increased mortality and severe adverse effects in PD patients (Ballard et al. 2015). The oral 5-HT2c receptor inverse agonist/antagonist, pimavanserin, received marketing authorization by the FDA in 2016. Pimavanserin intake resulted in a reduction of psychosis symptoms and prevention of relapse in PD psychosis without worsening of motor or cognitive functions. Notable adverse effects in studies mainly comprised headaches, constipation, edema, hallucinations, and urinary tract infections. Due to QTc prolongation in some patients, additional pharmacovigilance is required (Tariot et al. 2021; Abler et al. 2022; Cummings et al. 2014; Isaacson et al. 2021). Currently, two phase 4 studies for comparison of treatments with quetiapine or pimavanserin are recruiting in the US (NCT04373317, NCT05590637). In addition, there is evidence of improvement of depressive symptoms in a completed phase 2 study (DeKarske et al. 2020). Its effect on impulse control disorders in PD will be assessed in another phase 2 study (NCT03947216). A further phase 2 study assesses the effects of ondansetron treatment, an antagonist at 5-HT3 receptors, on hallucinations (NCT04167813).
Cognitive impairment is common in PD with mild cognitive impairment being present in more than 10% of PD patients and increases over the course of the disease. PD with dementia is estimated to develop in more than 70 percent of patients after 10 years of disease progression (Litvan et al. 2011). Current standard for pharmacologic symptomatic therapy primarily consists of cholinesterase inhibitors. Other pathways currently under assessment in clinical trials include modulation of the NMDA receptor (phase 2: NCT04470037, NCT05318937), agonism at the beta-2-adrenoreceptor (phase 2: NCT04739423, NCT05104463), antagonism at the adenosine A2A receptor (phase 2: NCT05333549) and antioxidative effects (NCT05084365). An oral formulation of ANAVEX2-73 (blarcamesine), modulating the sigma-1-receptor, was found to improve motor and cognitive functions in a phase 2 trial and its extension study (NCT03774459, NCT04575259, Barwicki 2022, 2023). Furthermore, infusions of GRF6021, a human plasma protein fraction, displayed satisfactory safety profile and improved quality of live and cognitive functions (phase 2: NCT03713957, Rawner 2021).
Constipation is an early and common non-motor symptom in PD. ENT-1, an oral compound inhibiting formation of α-synuclein aggregates, reduced constipation and additionally improved cognitive and psychosis-related outcome measures (Camilleri et al. 2022). Further currently assessed substances for constipation include pyridostigmine (NCT05603715) and probiotic interventions (NCT05204641), also evaluating the potential impact of the latter on disease progression or depression and anxiety (NCT03575195, NCT03968133, NCT04871464, NCT05568498, NCT05576818).
After improving insomnia in patients with Alzheimer’s disease (Herring et al. 2020), orally administered suvorexant, inducing antagonism at the orexin receptor, is investigated for its effect on insomnia (NCT02729714). In addition, continuous subcutaneous application of apomorphine was shown to improve sleep disturbances in PD patients with motor fluctuations (Cock et al. 2022). Oral GABAB agonist valiloxybate is assessed for possible improvements in excessive daytime sleepiness and sleep quality with already promising results in healthy subjects (NCT05056194, Xiang and Rappaport 2021).
Due to the complex involvement of the dopaminergic system in pain, subcutaneous apomorphine (NCT04879134) or oral opicapone (NCT04986982, Chaudhuri et al. 2022) will be investigated for the treament of pain in PD patients. As previous studies in PD could not establish a significant effect, another phase 2 study is evaluating the use of cannabis oil preparations in PD patients (NCT03639064).
Disease modification
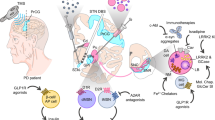
Although symptomatic treatment has strikingly improved over the last decades, DMT bear the hope to modulate disease progression at its roots and would therefore alter our therapeutic landscape significantly. In the following, we will give a brief overview on disease-modifying targets currently investigated and then focus on promising therapies in advanced stages of development (Table 2). Some approaches for disease modification target specific proteins (LRRK2 or GCase) or organelles (mitochondria or lysosomes), while others focus on the reduction of aSyn pathology, broadly accepted to be relevant in the majority of sporadic PD patients, by influencing aSyn-production, turnover, aggregation, cell-to-cell propagation, or else influence downstream mechanisms such as neuronal survival or immunomodulation (Vijiaratnam et al. 2021).
Alpha-synuclein
Aggregated aSyn exerts its toxic effects via reducing synaptic vesicles motility, promoting lysosomal and mitochondrial dysfunction, and impairing protein transport from the endoplasmic reticulum to the Golgi apparatus and autophagy (Wong and Krainc 2017). Therefore, DMTs that focus on reducing or mitigating the aSyn burden have been of highest interest. One of the oldest approaches for disease modification by targeting aSyn pathology focuses on specifically engaging aggregated aSyn and interfering with cell-to-cell transmission by immunotherapy. In 2005, first beneficial histopathological effects of immunization with human aSyn have been proposed (Masliah et al. 2005) and many follow-up studies provided evidence that either immunization against aSyn or treatment with aSyn-specific monoclonal antibodies are suitable to reduce phenotypic and neuropathological alterations in in vitro and in vivo PD models (Masliah et al. 2011; Shahaduzzaman et al. 2015; Schofield et al. 2019; Höllerhage et al. 2022). In the case of active immunization (vaccination) two approaches have advanced to clinical trials. On the one hand, PD01A and PD03A both completed phase 1 in PD patients (NCT01568099; NCT02267434) and ACI-7104, an optimized formulation of PD01A, was announced to proceed to phase 2 (AC Immune SA 2021). On the other hand, UB-312, having completed phase 1 (NCT04075318) just recently (Yu et al. 2022), proceeded to phase 2 in patients with α-synucleinopathies (NCT05634876) and will start patient recruitment in 2023. PD01A and UB-312 presumably act in a comparable way by providing a C-terminal epitope and generating an immune response specific against aSyn oligomers. In terms of passive immunotherapy, results have been inconclusive so far. Cinpanemab (BIIB054), an N-terminal, aggregate-specific antibody, was not able to provide evidence for clinical efficacy in phase 2 (change of MDS-UPDRS I-III after 52 weeks; NCT03318523) and its development has been terminated (Lang et al. 2022). Prasinezumab (PRX002), a C-terminal antibody, missed its primary endpoint in the phase 2 trial (change of MDS-UPDRS I-III after 52 weeks; NCT03100149), yet showed a trend to slow decline in motor function (MDS-UPDRS part III). Therefore, efficacy of prasinezumab is currently investigated in the open-label extension of this trial and in an additional phase 2b study over 18 months (NCT04777331) (Pagano et al. 2021, 2022). Two additional antibodies, MEDI1341 (also known as TAK-341) and Lu AF82422 completed phase 1 trials in healthy volunteers and patients with PD (NCT04449484 and NCT03611569, respectively), both being C-terminal and binding monomeric and aggregated aSyn. Both antibodies are currently investigated in phase 2 trials in patients with MSA (NCT05526391, NCT05104476, respectively).
aSyn-selective antisense oligonucleotides have been shown to reduce aSyn expression in a mouse model of PD and enhanced neurotransmitter release (Alarcón-Arís et al. 2018). A phase 1 trial currently investigates intrathecal administration of antisense oligonucleotide ION464 targeting aSyn-coding mRNA (SNCA mRNA) in patients with Multiple System Atrophy (MSA), another α-synucleinopathy, and might therefore be potentially relevant for PD patients in the future (NCT04165486).
Very recently, two small molecules with potential to inhibit the formation of presumably toxic aSyn oligomers have been investigated: anle138b, an orally administered diphenyl-pyrazole, potent to modulate aSyn aggregate formation and even to disintegrate aSyn oligomers, has shown a favorable safety and tolerability profile in healthy subjects (NCT04208152; Levin et al. 2022) and has completed the recruitment process in a phase 1 trial in PD patients (NCT04685265), with results expected in 2023. In parallel, NPT200-11, also known as UCB0599, binds to the C-terminus of the aSyn monomer and inhibits dimer formation leading to reduced cortical aSyn pathology and neuroinflammation while improving motor function in a mouse model overexpressing human aSyn (Wrasidlo et al. 2016; Price et al. 2018). Oral UCB0599 has provided reasonable safety/tolerability profile with favorable pharmacokinetics in PD patients (NCT04875962; Smit et al. 2022) and has advanced to a phase 2 trial (NCT04658186) with an extension study planned (NCT05543252).
Neuroinflammation
A pro-inflammatory immune phenotype, characterized by innate and adaptive immune cell activation, increase of circulating pro-inflammatory cytokines, blood–brain barrier permeability and peripheral immune cell infiltration of the central nervous system has been identified as hallmark of PD. Immunomodulatory or anti-inflammatory approaches may therefore represent promising disease-modifying targets (Tansey et al. 2022; Caldi Gomes et al. 2022). Some anti-inflammatory efforts have failed to provide satisfactory efficacy to date (e.g., NSAIDs (Poly et al. 2019), simvastatin (Stevens et al. 2022), verdiperstat (Biohaven Pharmaceutical Holding Company Ltd. 2021)). Modulation of microglia activation may represent another promising target. Fasudil, a neuroprotective Rho-kinase inhibitor (Tatenhorst et al. 2016) reducing pro-inflammatory cytokines (Zhao et al. 2015) and regulating microglia activation (Zhang et al. 2013), just recently completed recruitment as DMT for amyotrophic lateral sclerosis (ALS, NCT03792490) and will be evaluated in its oral formulation in patients with tauopathies (NCT04734379) and PD patients (EudraCT: 2021-003879-34; authors note). Furthermore, the disease-modifying potential of azathioprine, which reduces T and B lymphocyte proliferation and therefore attenuates inflammatory response, will be evaluated in more rapidly progressing PD patients (EudraCT: 2018-003089-14; Greenland et al. 2020). Patients will be selected by a previously established prognostic model, based on age and clinical evaluation (higher UPDRS-ME axial score and lower animal fluency score; Velseboer et al. 2016). GLP-1 receptor agonists, approved as treatment for type 2 diabetes, have shown promising preclinical results by reducing neuroinflammation (Chen et al. 2018) and decreasing aSyn burden (Zhang et al. 2019). Different GLP-1 receptor agonists are currently evaluated with exenatide being the most advanced. Exenatide-PD3 (NCT04232969) will investigate an extended-release formulation of subcutaneous exenatide over 2 years in PD patients with differences in MDS-UPDRS part III as primary outcome.
Mitochondria
Two relevant genes for the development of early onset PD are Parkin and PINK1, and mutations result in recessively inherited PD, both leading to compromised neuronal ability to remove damaged mitochondria (mitophagy), leading to increased amount of dysfunctional mitochondria, release of mitochondrial damage-associated molecular patterns (mitoDAMPs), and neuroinflammation (Borsche et al. 2021). While two DMTs targeting mitochondrial dysfunction (inosine and pioglitazone) did not alter disease progression in PD in phase 2 and 3 trials (NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators 2015; Schwarzschild et al. 2021), ursodeoxycholic acid is currently investigated in a phase 2 trial (NCT03840005). Notably, bile acid tauroursodeoxycholic acid (TUDCA) in combination with sodium phenylbutyrate has been evaluated as neuroprotective agent in Alzheimer’s disease (Becky Gohsler 2021) and was just recently approved by the FDA for patients with Amyotrophic lateral sclerosis (Paganoni et al. 2022).
Leucine-rich repeat kinase 2 (LRRK2)
Another promising target for DMTs is leucine-rich repeat kinase 2 (LRRK2). The LRRK2 Gly2019Ser mutation is the most common cause of autosomal-dominant PD and common variants in LRRK2 modulate the risk to develop PD. Inhibitors of LRRK2 promote autophagy (Manzoni et al. 2013), reduce neuroinflammation, and are therefore promising candidates for preventing neurodegeneration (Daher et al. 2015). DNL151 (also known as BIIB122), an orally administered LRRK2 inhibitor, has passed phase 1 and its disease-modifying efficacy is currently evaluated in PD patients with known LRRK2 mutation in up to 180 weeks (NCT05418673) and sporadic PD patients without LRRK2 mutation in up to 144 weeks (NCT05348785). In parallel, BIIB094, an intrathecally administered LRRK2 antisense oligonucleotide, is tested for safety and tolerability in sporadic PD patients in 18 global study centers (NCT03976349).
Glucocerebrosidase (GBA)
In the European population, the most relevant genetic risk factor for PD are variants of GBA, the gene encoding for glucocerebrosidase. Mutations increase PD risk ranging from threefold to 15-fold, depending on the variant (Day and Mullin 2021). While homozygous or compound heterozygous GBA mutations lead to the lysosomal storage disorder Gaucher’s disease, ~ 10% of European PD patients harbor GBA variants (Skrahina et al. 2021). Mutated glucocerebrosidase disrupts glycosphingolipid homoeostasis, leads to lysosomal dysfunction, and aSyn aggregation (Do et al. 2019). A glucocerebrosidase gene therapy (PR001) administered into the cisterna magna has been of highest interest for treatment of Gaucher’s disease (NCT04411654, NCT05487599), but will also be evaluated in PD patients with at least one known GBA mutation (NCT04127578). Efforts to increase the activity of glucocerebrosidase have yielded promising results. Ambroxol, acting as a chaperone of the lysosomal enzyme glucocerebrosidase, displayed good target engagement, increased glucocerebrosidase and aSyn levels in CSF, and lead to improvement in MDS-UPDRS part III in an open-label study in patients with and without GBA mutation (NCT02941822). Ambroxol has furthermore advanced into a multicenter, placebo-controlled phase 3 trial in PD patients (NCT05778617).
Regenerative or restorative therapies
At the borderline between symptomatic and disease-modifying therapies, there are two approaches to counteract or even reverse neuron loss in the brains of PD patients, but without interfering with the underlying pathology. Early works on cell replacement therapies by transplantation of dopaminergic neurons derived from fetal mesencephalon into the putamen displayed promising results including long-term survival of grafted neurons (Olanow et al. 2009) yet double-blind trials showed only mild clinical effects in younger or less severely affected patients (Olanow et al. 2003; Freed et al. 2001). This approach, which relies on fetal tissue, presents many challenges for translation to large patient numbers. Recent approaches try to overcome this by utilizing dopaminergic progenitor cells derived from embryonic stem cell lines as grafts (NCT05635409, NCT04802733). Another approach to prevent loss of dopaminergic terminals in the striatum and neuron loss in the substantia nigra is by administration of neurotrophic factors (e.g., glial cell-derived neurotrophic factor (GDNF), neurturin), known to activate signaling cascades critical for neuron survival and neurite outgrowth (Olanow et al. 2015). However, bioavailability in the brain is a major concern for tropic factors requiring an intrathecal or surgical administration. Previous controlled clinical trials failed to translate promising preclinical data into clinical settings (Lang et al. 2006; Olanow et al. 2015). Currently a GDNF gene transfer approach using adeno-associated viral vectors (AAV2-GDNF) is evaluated in open-label phase 1 trials in PD patients (NCT04167540, NCT01621581) with results indicating an acceptable safety profile of the MRI-guided putaminal infusion (Rocco et al. 2022).
Trial design and biomarkers
Aside from the selection of interventional targets in representative preclinical models, adequate trial designs with sufficient duration, representative measures of clinical disease progression, and a minimum of confounding by symptomatic effects will help to overcome past failures in disease-modifying trials (Vijiaratnam et al. 2021). One promising trial design is the delayed-start design: in a RCT investigating disease-modifying effect of rasagiline (NCT00256204), patients with PD were randomly assigned to either rasagiline (1 mg or 2 mg) for 72 weeks or placebo treatment for 36 weeks, followed by rasagiline (1 mg or 2 mg) for 36 weeks. This trial setup enables to distinguish between symptomatic effects (difference in symptom severity is only present before switch of the delayed-start group to verum) and disease-modifying effects (differences in symptom severity continue to be present to the end of the trial). Biomarkers for target engagement have been established but are not applied in a wider scale. For example, antibodies against aSyn were shown to reduce unbound serum aSyn by up to 97% (Jankovic et al. 2018) and LRRK2 inhibitor BIIB122 reduced concentrations of LRRK2 and phosphorylation (pT73) of Rab10 (Jennings et al. 2023), a direct substrate of LRRK2. So far, there are no biomarkers of histopathological disease progression available as readout for clinical trials of disease modification; however, promising approaches will be covered in the biomarker section of this article.
One pill fits all versus precision medicine
In current medical practice, we acknowledge the variability of clinical presentations of PD patients and tailor symptomatic treatment accordingly. Numerous studies attempting to identify PD subtypes have focused on clinical markers, but our increasing knowledge of disease heterogeneity suggests that molecular heterogeneity should be considered as well (Mestre et al. 2021; Espay et al. 2017a). Identification of both clinical and molecular subgroups could enable tailored individual therapeutic solutions (Cholerton et al. 2016; Espay et al. 2017b; Titova and Chaudhuri 2017). Molecular stratification can be performed based on (a) pathogenic mutations that affect a particular molecular pathway, (b) the identification of clusters of common genetic variants in genes associated with specific disease-associated pathways, or (c) biomarkers that indicate the level of function/dysfunction of disease-associated pathways in sporadic PD patients.
Several monogenic causes for PD have been identified (Blauwendraat et al. 2020). These findings have for example implicated dysfunction of mitophagy and autophagy in the pathogenesis of PD (Nguyen et al. 2019; Singleton and Hardy 2019). Mendelian PD is thought to potentially serve as a model for the identification of biomarkers representing underlying pathophysiology and for the development of targeted therapies (Hockey et al. 2015; Mortiboys et al. 2013; Peterschmitt et al. 2022). Efforts like the Rostock International PD (ROPAD) and the LRRK2/Luebeck International PD (LIPAD) studies aim at genetic classification and deep phenotyping of PD patients and healthy carriers of pathogenic variants (Skrahina et al. 2021; Usnich et al. 2021). Initial ROPAD data showed a remarkable genetic diagnostic yield with the identification of disease-associated variants in approximately 14% of 1360 screened PD patients. Variants in GBA (in 8.5% of all patients screened), LRRK2 (3.1%), and compound heterozygous PRKN variants (0.8%) were identified most frequently. These patients could be included in clinical trials focusing on genetic subgroups, for example with the LRRK2-Inhibitor BIIB122 (NCT05418673) (see section on “disease modification”).
In most PD patients, however, a disease-causing mutation cannot be found. Nevertheless, common genetic variants modify the risk to develop PD with smaller effect sizes. Identified common variants have linked PD to numerous pathways including lysosomal function, the immune system and metabolism (Fernández-Santiago and Sharma 2022). Polygenic risk scores (PRS) compile common low-risk variants and have been shown to be associated with disease risk, age of onset and disease progression (Dehestani et al. 2021; Paul et al. 2018; Pihlstrøm et al. 2022). For patient stratification, it may also be possible to use pathway-specific PRS, which consist of variants related to particular pathways, such as mitochondrial PRS and autophagy-lysosomal PRS (Bandres-Ciga et al. 2020; Billingsley et al. 2019; Dehestani et al. 2022). While pathway-specific PRS may be indicative of an underlying disease process, it seems more intuitive to use direct markers of current pathway function (e.g., 31Phosphorus-Magnetic resonance spectroscopy imaging for mitochondrial dysfunction in PD patients, NCT03815916) to define clinically relevant and mechanistically anchored disease subgroups (Rosen and Zeger 2019; Prasuhn et al. 2020).
Mitochondria-associated blood biomarkers do not necessarily reflect mitochondrial dysfunction in neurons or, more specifically, dopaminergic neurons. Expression patterns often are tissue-specific and mtDNA copy number in blood has been shown to be very variable (Pyle et al. 2016; Davis et al. 2020; Müller-Nedebock et al. 2022). Imaging studies using phosphorus magnetic resonance spectroscopy (31P-MRS) to explore in vivo mitochondrial function in PD patients have shown contrasting results to date (Dossi et al. 2019). A seemingly promising approach is the investigation of skin fibroblasts, which can be directly patient-derived and studied as individual readout in vitro. Using a combination of cellular assays, RNA-sequencing based pathway analysis and genotyping, distinct subgroups of PD patients with mitochondrial and lysosomal dysfunction could be identified (Carling et al. 2020). Other biomarkers related to autophagy and lysosomal function have shown inconsistent results (Xicoy et al. 2019). In sporadic PD, reduced heatshock cognate-70 (Hsc70) levels in peripheral blood mononuclear cells (PBMC) were suggested as a marker of chaperone-mediated autophagy dysfunction, but large-scale studies are still missing (Papagiannakis et al. 2015; Sala et al. 2014). Specific GBA variants are associated with variable but consistent reduction in glucocerebrosidase (GCase) activity (Alcalay et al. 2015; Lerche et al. 2021). In sporadic PD, several studies demonstrated a significant reduction of GCase activity while some did not find a difference compared to controls (Atashrazm et al. 2018; van Dijk et al. 2013; Xicoy et al. 2019). Therapies aiming to enhance GCase function in GBA-PD could also be beneficial for sporadic PD patients with reduced GCase activity (Heijer et al. 2021). Inflammatory biomarkers such as IL-6, IL-10, IL-1β, tumor necrosis factor and others have been shown to be increased in blood and CSF of patients with PD (Harms et al. 2021; Qin et al. 2016; Zimmermann and Brockmann 2022). The extent of inflammation is associated with the clinical presentation in patients with LRRK2-associated PD and disease progression in sporadic PD (Brockmann et al. 2017; Williams-Gray et al. 2016). Inflammatory markers in CSF and blood often are not correlated, change over time and are also present in patients with other neurodegenerative disorders and, therefore, not disease specific (Zimmermann and Brockmann 2022). Still, PD patients with above average contribution of neuroinflammation to their disease may be the ones benefiting most from immunomodulatory therapies.
Overall, clinical and molecular subgroups should be more intensively used for the design of targeted clinical trials and could result in more specific therapeutic options for PD patients.
Device-assisted therapeutic monitoring
The success of symptomatic and disease-modifying therapy is based on regular assessment of symptom burden, side effects, and treatment adherence. Still, 40% of patients with PD in Europe and the US are not evaluated by neurologists or movement disorder specialists (Dorsey et al. 2018), and access to specialized care is particularly limited in rural areas or developing countries (Dorsey and Bloem 2018). In addition, the evaluation of symptoms of PD patients is largely limited to short episodes of in-clinic visits and outpatient consultations as well as patient-completed symptom diaries and subjective reports. Relevant aspects might not be captured in these contacts due to symptom fluctuations, rare occurrences, or relevant differences between supervised assessments and symptoms in daily life (Warmerdam et al. 2020).
Device-assisted digital assessments during inpatients visits can help objectify clinical evaluation, measure treatment response, and help overcome interrater variations. For example, digital motion biomarkers enable measurement of discreet movement disturbances not visible during routine examination (e.g., smoothness of gait and jerk of foot, Kuhner et al. 2019). In parallel to cardinal motor features, machine learning-based speech analyses were able to identify early and mid-stage PD patients with high accuracy (Suppa et al. 2022). To quantify therapy effects, markerless motion capture systems help improve motivation and outcome during neurorehabilitation (Knippenberg et al. 2017).
In addition, remote sensor-based assessments are becoming increasingly important to measure motor symptoms in a natural environment, and real-word studies have shown feasibility and acceptance of tools such as wearable sensors, smartphone apps, and smartwatches (Adams et al. 2021; Bendig et al. 2022; Powers et al. 2021). Most wearable sensors contain accelerometers and gyroscopes and are placed on one or more locations on variable parts of the body such as trunk, upper, or lower extremities. Depending on the device, measurements of bradykinesia, dyskinesia, tremor, gait, falls, or overall physical activity are possible (Ancona et al. 2022) Positive effects on clinical outcome have been shown when experts were supported by wearable sensors in their clinical decision making (Woodrow et al. 2020; Isaacson et al. 2019). Moreover, wearable sensors have also been included in recent trials to measure at home functioning as a secondary outcome (NCT04739423, NCT04380142). To establish the use of wearable sensors into the daily clinical routine, large-scale RCTs validating assessments in real world conditions and giving evidence of benefits of sensor-based assessments over the current clinical standards in terms of therapeutic effects, quality of life, or cost-effectiveness are needed (Del Din et al. 2021). Among others, the Movement Disorder Society has developed a roadmap for the implementation of digital outcome measures to overcome the current limitations (Espay et al. 2019).
Early diagnosis and biomarker development
There is a growing need for objective biomarkers that allow earlier diagnosis, the quantification of disease-relevant molecular processes, and treatment response for DMTs (The Parkinson Progression Marker Initiative (PPMI) 2011).
Clinical measures (e.g., MDS-UPDRS) provide an estimate of symptom severity and—used in the right setting—valid measure for clinical disease progression as outcome parameters in clinical studies of new DMTs. However, clinical scores are heavily biased by symptomatic effects, do not capture subclinical effects on molecular processes, or effects of potential DMTs in pre-symptomatic subjects. Some efforts in the detection of preclinical symptoms with have been made, and data suggest the potential of wearable accelerometer devices to identify prodromal PD (Schalkamp et al. 2022).
Structural and functional neuroimaging has been used in aiding diagnosis of unclear phenotypes as well as monitoring of therapeutic effects in drug trials as outcome measure. Structural and volumetric MRI findings are subtle in early PD and often not be detectable by conventional MRI. Morphometry analyses revealed reduced gray matter volumes in different cortical and subcortical regions to inconsistent extend. These findings enable differentiation between PD patients and controls or other neurodegenerative disorders with varying accuracy (Saeed et al. 2017). Functional neuroimaging with different radioligands by single photon emission computed tomography (SPECT), however, enables to quantify the integrity of the nigrostriatal system. Occurrence of early non-motor symptoms of PD such as hyposmia and constipation correlate with abnormal dopamine transporter (DAT) binding in 123Iβ-CIT SPECT imaging (Jennings et al. 2014). Reduction in DAT binding accompanies disease progression and correlates moderately with MDS-UPDRS scores (Simuni et al. 2018). DAT scan has shown feasible as secondary or exploratory outcome for trials investigating DMTs. In a trial comparing levodopa and pramipexole in early PD patients, patients who received pramipexole showed less reduction in 123Iβ-CIT uptake compared to the levodopa group, which also correlated with UPDRS scores (Parkinson Study Group 2002). Patients with normal DAT scans and early non-motor symptoms have been shown to be less likely to convert to PD than patients with changes in DAT scan (Batla et al. 2014; Lee et al. 2021). In addition, nigrostriatal integrity can also be evaluated by positron emission tomography (PET, e.g., by 18F-dopa), but PET imaging also harbors promising applications for measuring glucose metabolism (18F-FDG), microglia-mediated neuroinflammation (e.g., 11C-(R)-PK11195), and protein (e.g., aSyn) accumulation (Saeed et al. 2017; Capotosti 2022). With the development of novel imaging markers, in vivo measurements of pathophysiological hallmarks can accompany clinical findings in symptom severity and are crucial for further development of DMTs.
Liquid biomarkers that reflect key pathological hallmarks of PD such as intracellular aggregation and intercellular spread of pathological forms of aSyn (Goedert et al. 2013; Yang et al. 2022) allow stratification of patients by their molecular diagnosis and are important for the success of α-synucleinopathy targeted treatments. So far, the measurement of total aSyn concentrations in the cerebrospinal fluid (CSF) has been unsatisfactory for the diagnosis of PD or as disease progression marker (Ohrfelt et al. 2009; Mollenhauer et al. 2008, 2017; Compta et al. 2015; Majbour et al. 2016; Eusebi et al. 2017). Total aSyn levels likely do not reflect the complex pathophysiology of α-synucleinopathy (Tofaris 2022; Stefanis et al. 2019). A more accurate measurement of disease-specific aSyn was achieved by isolation of neuron-derived extracellular vesicles from serum (Jiang et al. 2020, 2021), even in combination with a seeded aggregation assay (Kluge et al. 2022). The measurement of disease-specific forms of aSyn such as oligomers or aggregates seem most promising to detect α-synucleinopathy even early in disease development. Real‐time quaking‐induced conversion (RT‐QuIC) exploits the aggregation properties of aSyn (Stefanis et al. 2019; Brandel et al. 2015). This assay can detect aSyn aggregation in the CSF of PD and dementia with Lewy bodies (DLB) patients with high sensitivity and specificity (Fairfoul et al. 2016; Groveman et al. 2018; Manne et al. 2019; Bongianni et al. 2019). Importantly, aSyn aggregation properties were detected in prodromal PD patients with clinical syndromes that preceded Parkinsonism or cognitive decline with high sensitivity of over 95%, meaning that RT-QuIC could potentially enable an early diagnosis (Rossi et al. 2020). In the large Parkinson's Progression Markers Initiative cohort of 1123 participants, 99% of PD patients with olfactory disfunction showed seeded aggregation and healthy controls were correctly identified with a specificity of 96% (Siderowf et al. 2023). RT‐QuIC has also been tested in symptomatic and non-symptomatic patients carrying a LRRK2 mutation, a common genetic risk factor for familial and sporadic PD, and seeding propensity was shown in a subset of patients (Garrido et al. 2019). RT-QuIC could therefore help to identify candidates to receive disease-modifying drugs even in an asymptomatic phase of disease. RT-QuIC from a nasal swab is especially promising since it enables less invasive biosampling (Perra et al. 2021; Stefani et al. 2021). Seeded aggregation assays may also be interesting to detect PD in subjects without initial evidence of dopamine deficit by imaging (Russo et al. 2021). Currently available RT-QuIC essays enable a qualitative analysis with either a negative or positive result. However, quantification of seeded oligomers in a positive sample could additionally help to monitor longitudinal changes over the course of a disease or in response to a treatment (Russo et al. 2021; Majbour et al. 2022).
Disease-unspecific biomarkers reflect downstream effects of pathology and could be proposed for the assessment of disease progression. While neurofilaments are not elevated in CSF and serum in idiopathic PD compared to age matched controls (Hansson et al. 2017; Marques et al. 2019), higher neurofilament levels in PD correlate with progressive motor dysfunction or cognitive decline (Lin et al. 2019; Aamodt et al. 2021). Neurofilament levels are increased in atypical parkinsonian syndromes compared to PD (Herbert et al. 2015; Hall et al. 2012; Hansson et al. 2017; Marques et al. 2019). Therefore, combining neurofilaments with α-synucleinopathy specific biomarkers could be used to stratify patients by individual rate of progression and diagnosis into treatment trials.
In patients with PD-associated dementia, Alzheimer’s like pathology including extracellular β-amyloid plaques as well as intracellular hyperphosphorylated tau (p-tau) deposition are seen in two-thirds of autopsied cases (Jellinger 2012; Smith et al. 2019). Levels of β-amyloid and p-tau181 in CSF have been shown to correlate with cognitive decline in nondemented people and patients with Alzheimer’s dementia, PD dementia, and vascular dementia (Fagan et al. 2007; Skillbäck et al. 2015). Low levels of β-amyloid in CSF have been associated with cognitive impairment in PD, while data for p-tau is inconsistent (Compta et al. 2009, 2013). Most studies show that plasma p-tau levels do not associate with cognitive decline in PD (Lin et al. 2018; Pagonabarraga et al. 2022; Batzu et al. 2022). However, higher plasma p-tau levels seem to predict Alzheimer’s disease pathology in dementia with Lewy bodies and PD dementia (Gonzalez et al. 2022). A prognostic value for cognitive decline in PD has been shown for CSF β-amyloid and neurofilaments (Siderowf et al. 2010; Bäckström et al. 2022). Predicting cognitive decline in PD can be a valuable tool for communicating prognosis to patients as well as clinical management and inclusion into clinical trials.
The accumulation of iron in the substantia nigra is a known feature in PD and increases in the disease course (Lhermitte et al. 1924; Dexter et al. 1989). Early studies on iron levels in PD provided inconclusive evidence on the ability to discriminate between healthy controls and patients with PD (Mariani et al. 2013; Medeiros et al. 2016; Lucio et al. 2019). An elemental cluster including six different elements (including iron) identified PD patients with high sensitivity and specificity (Maass et al. 2018). Further, the iron and ferritin CSF levels show inverse changes in a longitudinal cohort of patients with PD indicating their potential as a progression marker (Maass et al. 2021). Warranting further validation in independent cohorts, change of iron and ferritin levels in response to DMT might, therefore, be used as surrogate marker to evaluate effects on disease progression.
In ALS, NfL already is an established biomarker for disease progression and also predicts phenoconversion in pre-symptomatic mutation carriers (Benatar et al. 2018). This feature is used in the ATLAS trial (Benatar et al. 2022): ATLAS uses pre-symptomatic gene mutation carriers of SOD1 to identify people at risk and submit them to therapy with the antisense oligonucleotide Tofersen. Tofersen was shown to decrease SOD1 and NfL levels in the CSF of treated patients in phase 1–2 and 3 trials (Miller et al. 2020, 2022) although no advantage of decline in the clinical scores was shown within the 24-week follow-up of the phase 3 trial. For the initial studies, however, only symptomatic patients were included. ATLAS now aims at slowing the course of the disease once a threshold of NfL is reached and before clinical symptoms are present. While a biomarker with similar properties is lacking for PD, studies are conducted recruiting patients with premotor symptoms of the disease such as Rapid-eye-movement (REM) sleep behavior disorder (RBD). Even though RBD is not present in all PD patients and some patients develop RBD in later stages of the disease, RBD is considered as one of the earliest and most specific prodromal signs of an α-synucleinopathy (Miglis et al. 2021). Rasagiline (NCT05611372) and idebenone (NCT04152655) are currently assessed for their impact on the progression time from RBD to PD.
Taken together, there are several promising fluid-biomarker candidates covering different aspects of the disease. While aSyn might be a parameter for measuring the pathological hallmark of the disease, NfL, iron, and ferritin levels have the potential to predict the progression of the disease, while β-amyloid and potentially p-Tau are promising to forecast cognitive impairment. While CSF might be the most relevant biomaterial for neurodegenerative diseases and is helpful as diagnostic tool, evaluation of easily accessible biomaterial might be of special relevance for longitudinal assessment. All these biomarkers have the potential to provide a more accurate diagnosis and differentiate subtypes of PD, and therefore will ultimately benefit clinical trial recruitment as well as the selection and monitoring of new therapies. Discovery and validation of new biomarkers will be crucial in refining these processes and thereby aid development of new disease-modifying therapies. In addition, identification of biomarkers predicting phenoconversion even before prodromal signs are present and will be crucial for trials testing disease-modulating substances early in the course of the disease.
Conclusion
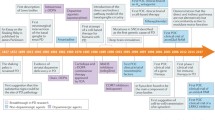
The therapeutic landscape in PD is highly dynamic. Although symptomatic treatments already today allow very good symptom control in earlier disease stages, more advanced stages of PD are still challenging. A big medical need exists in the area of non-motor symptoms, but it is contrasted by a vibrant clinical trial landscape. The development of disease-modifying therapies will fundamentally change the therapeutic landscape in the future. In addition, it can be assumed that with the increasing use of biomarkers, therapy will be more targeted to the individual patient. This will pave the way for future therapies to be applied not only in symptomatic patients, but also to develop therapeutic strategies that start at the pre-symptomatic stage and can thus delay the onset of the disease and mitigate its progression (Fig. 1).
A PD therapy currently starts after the development of motor symptoms and diagnosis is made based on clinical symptoms. Symptomatic treatment (ST) is started and initially leads to good symptom control. With increasing disease progression and symptom burden, ST is adapted. B In the future, biomarker-based risk stratification will help identify people at risk with subclinical manifestations. Disease-modifying therapy (DMT) will be started in a premotor or prodromal phase, e.g., in a progression marker-based subset of people with high risk of phenoconversion. Clinical PD diagnosis could include a combination of clinical symptoms and biomarkers. Symptomatic therapy (ST) will be started and adapted by symptom severity as experienced by the patient and established clinical scales with the help of digital health applications. DMT will be adapted to the disease stage using biomarker-based stratification
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed for this article.
Abbreviations
- aSyn:
-
Alpha-synuclein
- CSAI:
-
Continuous subcutaneous apomorphine infusion
- DBS:
-
Deep brain stimulation
- DMT:
-
Disease-modifying therapy
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- GBA:
-
Glucocerebrosidase
- GPI:
-
Globus pallidus internus
- LCIG:
-
Levodopa–carbidopa intestinal gel
- LECIG:
-
Levodopa–entacapone–carbidopa intestinal gel
- LRRK2:
-
Leucine-rich repeat kinase 2
- MRgFUS:
-
MR-guided focused ultrasound
- PD:
-
Parkinson’s disease
- PRS:
-
Polygenic risk scores
- RCTs:
-
Randomized controlled trials
- SSRI:
-
Selective serotonin reuptake inhibitor
- SNRI:
-
Serotonin norepinephrine reuptake inhibitor
- TCA:
-
Tricyclic antidepressant
- STN:
-
Subthalamic nucleus
- VIM:
-
Nucleus ventralis intermedius thalami
References
Aamodt WW, Waligorska T, Shen J, Tropea TF, Siderowf A, Weintraub D, Grossman M, Irwin D, Wolk DA, Xie SX, Trojanowski JQ, Shaw LM, Chen-Plotkin AS (2021) Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov Disord 36(12):2945–2950
Abler V, Brain C, Ballard C, Berrio A, Coate B, Espay AJ (2022) Motor- and cognition-related safety of pimavanserin in patients with Parkinson’s disease psychosis. Front Neurol 13:919778
AC Immune SA (2021) AC Immune Announces Strategic Acquisition of Industry-leading Parkinson’s Disease Vaccine Candidate and Equity Investment Led by Athos Service GmbH. https://ir.acimmune.com/news-releases/news-release-details/ac-immune-announces-strategic-acquisition-industry-leading. Accessed 06 Jan 2023
Adams JL, Dinesh K, Snyder CW, Xiong M, Tarolli CG, Sharma S, Dorsey ER, Sharma G (2021) A real-world study of wearable sensors in Parkinson’s disease. Npj Parkinsons Dis 7(1):106
Alarcón-Arís D, Recasens A, Galofré M, Carballo-Carbajal I, Zacchi N, Ruiz-Bronchal E, Pavia-Collado R, Chica R, Ferrés-Coy A, Santos M, Revilla R, Montefeltro A, Fariñas I, Artigas F, Vila M, Bortolozzi A (2018) Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: potential therapy for Parkinson’s disease. Mol Ther 26(2):550–567
Alcalay RN, Levy OA, Waters CC, Fahn S, Ford B, Kuo S-H, Mazzoni P, Pauciulo MW, Nichols WC, Gan-Or Z, Rouleau GA, Chung WK, Wolf P, Oliva P, Keutzer J, Marder K, Zhang X (2015) Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain 138(Pt 9):2648–2658
Ancona S, Faraci FD, Khatab E, Fiorillo L, Gnarra O, Nef T, Bassetti CLA, Bargiotas P (2022) Wearables in the home-based assessment of abnormal movements in Parkinson’s disease: a systematic review of the literature. J Neurol 269(1):100–110
Antonini A, Moro E, Godeiro C, Reichmann H (2018a) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33(6):900–908
Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, Onuk K, Odin PLA (2018b) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin 34(12):2063–2073
Atashrazm F, Hammond D, Perera G, Dobson-Stone C, Mueller N, Pickford R, Kim WS, Kwok JB, Lewis SJG, Halliday GM, Dzamko N (2018) Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci Rep 8(1):15446
Bäckström D, Granåsen G, Mo SJ, Riklund K, Trupp M, Zetterberg H, Blennow K, Forsgren L, Domellöf ME (2022) Prediction and early biomarkers of cognitive decline in Parkinson disease and atypical parkinsonism: a population-based study. Brain Commun 4(2):fcac040
Ballard C, Isaacson S, Mills R, Williams H, Corbett A, Coate B, Pahwa R, Rascol O, Burn DJ (2015) Impact of current antipsychotic medications on comparative mortality and adverse events in people with parkinson disease psychosis. J Am Med Dir Assoc 16(10):898.e1–7
Bandres-Ciga S, Saez-Atienzar S, Kim JJ, Makarious MB, Faghri F, Diez-Fairen M, Iwaki H, Leonard H, Botia J, Ryten M, Hernandez D, Gibbs JR, Ding J, Gan-Or Z, Noyce A, Pihlstrom L, Torkamani A, Soltis AR, Dalgard CL, Scholz SW, Traynor BJ, Ehrlich D, Scherzer CR, Bookman M, Cookson M, Blauwendraat C, Nalls MA, Singleton AB (2020) Large-scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol 140(3):341–358
Barwicki AJ (2022) Anavex Life Sciences Announces Presentation of Phase 2 Clinical Biomarker Data from PDD study. https://www.anavex.com/post/anavex-life-sciences-announces-presentation-of-phase-2-clinical-biomarker-data-from-pdd-study. Accessed 14 Apr 2023
Barwicki AJ (2023) ANAVEX®2-73 (Blarcamesine) shows clinical benefit in long-term 48week phase 2 extension study in PDD. https://www.anavex.com/post/anavex-2-73-blarcamesine-shows-clinical-benefit-in-long-term-48week-phase-2-extension-study-in-pdd. Accessed 14 Apr 2023
Batla A, Erro R, Stamelou M, Schneider SA, Schwingenschuh P, Ganos C, Bhatia KP (2014) Patients with scans without evidence of dopaminergic deficit: a long-term follow-up study. Mov Disord 29(14):1820–1825
Batzu L, Rota S, Hye A, Heslegrave A, Trivedi D, Gibson LL, Farrell C, Zinzalias P, Rizos A, Zetterberg H, Chaudhuri KR, Aarsland D (2022) Plasma p-tau181, neurofilament light chain and association with cognition in Parkinson’s disease. Npj Parkinsons Dis 8(1):154
Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A (2018) Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol 84(1):130–139
Benatar M, Wuu J, Andersen PM, Bucelli RC, Andrews JA, Otto M, Farahany NA, Harrington EA, Chen W, Mitchell AA, Ferguson T, Chew S, Gedney L, Oakley S, Heo J, Chary S, Fanning L, Graham D, Sun P, Liu Y, Wong J, Fradette S (2022) Design of a randomized, placebo-controlled, phase 3 trial of tofersen initiated in clinically presymptomatic SOD1 variant carriers: the ATLAS study. Neurotherapeutics 19(4):1248–1258
Bendig J, Wolf A-S, Mark T, Frank A, Mathiebe J, Scheibe M, Müller G, Stahr M, Schmitt J, Reichmann H, Loewenbrück KF, Falkenburger BH (2022) Feasibility of a Multimodal telemedical intervention for patients with Parkinson’s disease-A pilot study. J Clin Med 11(4):1074
Billingsley KJ, Barbosa IA, Bandrés-Ciga S, Quinn JP, Bubb VJ, Deshpande C, Botia JA, Reynolds RH, Zhang D, Simpson MA, Blauwendraat C, Gan-Or Z, Gibbs JR, Nalls MA, Singleton A, Ryten M, Koks S (2019) Mitochondria function associated genes contribute to Parkinson’s disease risk and later age at onset. Npj Parkinsons Dis 5:8
Biohaven Pharmaceutical Holding Company Ltd. (2021) Biohaven Provides Update On Phase 3 Trial And Multiple System Atrophy (MSA) Program. https://www.prnewswire.com/news-releases/biohaven-provides-update-on-phase-3-trial-and-multiple-system-atrophy-msa-program-301385193.html. Accessed 04 Jan 2023
Blauwendraat C, Nalls MA, Singleton AB (2020) The genetic architecture of Parkinson’s disease. Lancet Neurol 19(2):170–178
Bomasang-Layno E, Fadlon I, Murray AN, Himelhoch S (2015) Antidepressive treatments for Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 21(8):833–842 (discussion 833)
Bond AE, Shah BB, Huss DS, Dallapiazza RF, Warren A, Harrison MB, Sperling SA, Wang X-Q, Gwinn R, Witt J, Ro S, Elias WJ (2017) Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol 74(12):1412–1418
Bongianni M, Ladogana A, Capaldi S, Klotz S, Baiardi S, Cagnin A, Perra D, Fiorini M, Poleggi A, Legname G, Cattaruzza T, Janes F, Tabaton M, Ghetti B, Monaco S, Kovacs GG, Parchi P, Pocchiari M, Zanusso G (2019) α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann Clin Transl Neurol 6(10):2120–2126
Borsche M, Pereira SL, Klein C, Grünewald A (2021) Mitochondria and Parkinson’s disease: clinical, molecular, and translational aspects. J Parkinsons Dis 11(1):45–60
Brandel J-P, Corbillé A-G, Derkinderen P, Haïk S (2015) La maladie de Parkinson est-elle une maladie à prion? (Is Parkinson’s disease a prion disease?). Revue Neurologique 171(12):812–824
Brockmann K, Schulte C, Schneiderhan-Marra N, Apel A, Pont-Sunyer C, Vilas D, Ruiz-Martinez J, Langkamp M, Corvol J-C, Cormier F, Knorpp T, Joos TO, Bernard A, Gasser T, Marras C, Schüle B, Aasly JO, Foroud T, Marti-Masso JF, Brice A, Tolosa E, Berg D, Maetzler W (2017) Inflammatory profile discriminates clinical subtypes in LRRK2-associated Parkinson’s disease. Eur J Neurol 24(2):427-e6
Caldi Gomes L, Galhoz A, Jain G, Roser A-E, Maass F, Carboni E, Barski E, Lenz C, Lohmann K, Klein C, Bähr M, Fischer A, Menden MP, Lingor P (2022) Multi-omic landscaping of human midbrains identifies disease-relevant molecular targets and pathways in advanced-stage Parkinson’s disease. Clin Transl Med 12(1):e692
Camilleri M, Subramanian T, Pagan F, Isaacson S, Gil R, Hauser RA, Feldman M, Goldstein M, Kumar R, Truong D, Chhabria N, Walter BL, Eskenazi J, Riesenberg R, Burdick D, Tse W, Molho E, Robottom B, Bhatia P, Kadimi S, Klos K, Shprecher D, Marquez-Mendoza O, Hidalgo G, Grill S, Li G, Mandell H, Hughes M, Stephenson S, Vandersluis J, Pfeffer M, Duker A, Shivkumar V, Kinney W, MacDougall J, Zasloff M, Barbut D (2022) Oral ENT-01 targets enteric neurons to treat constipation in Parkinson disease: a randomized controlled trial. Ann Intern Med 175(12):1666–1674
Capotosti F (2022) Discovery of [18F]ACI-12589: a novel and promising pet-tracer for alpha-synuclein. International Conference on Alzheimer’s and Parkinson’s Disease
Carling PJ, Mortiboys H, Green C, Mihaylov S, Sandor C, Schwartzentruber A, Taylor R, Wei W, Hastings C, Wong S, Lo C, Evetts S, Clemmens H, Wyles M, Willcox S, Payne T, Hughes R, Ferraiuolo L, Webber C, Hide W, Wade-Martins R, Talbot K, Hu MT, Bandmann O (2020) Deep phenotyping of peripheral tissue facilitates mechanistic disease stratification in sporadic Parkinson’s disease. Prog Neurobiol 187:101772
Chaudhuri KR, Odin P, Ferreira JJ, Antonini A, Rascol O, Kurtis MM, Storch A, Bannister K, Soares-da-Silva P, Costa R, Magalhães D, Rocha JF (2022) Opicapone versus placebo in the treatment of Parkinson’s disease patients with end-of-dose motor fluctuation-associated pain: rationale and design of the randomised, double-blind OCEAN (OpiCapone Effect on motor fluctuations and pAiN) trial. BMC Neurol 22(1):88
Chen S, Yu S-J, Li Y, Lecca D, Glotfelty E, Kim HK, Choi H-I, Hoffer BJ, Greig NH, Kim DS, Wang Y (2018) Post-treatment with PT302, a long-acting Exendin-4 sustained release formulation, reduces dopaminergic neurodegeneration in a 6-Hydroxydopamine rat model of Parkinson’s disease. Sci Rep 8(1):10722
Cholerton B, Larson EB, Quinn JF, Zabetian CP, Mata IF, Keene CD, Flanagan M, Crane PK, Grabowski TJ, Montine KS, Montine TJ (2016) Precision medicine: clarity for the complexity of dementia. Am J Pathol 186(3):500–506
Compta Y, Martí MJ, Ibarretxe-Bilbao N, Junqué C, Valldeoriola F, Muñoz E, Ezquerra M, Ríos J, Tolosa E (2009) Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord 24(15):2203–2210
Compta Y, Pereira JB, Ríos J, Ibarretxe-Bilbao N, Junqué C, Bargalló N, Cámara A, Buongiorno M, Fernández M, Pont-Sunyer C, Martí MJ (2013) Combined dementia-risk biomarkers in Parkinson’s disease: a prospective longitudinal study. Parkinsonism Relat Disord 19(8):717–724
Compta Y, Valente T, Saura J, Segura B, Iranzo Á, Serradell M, Junqué C, Tolosa E, Valldeoriola F, Muñoz E, Santamaria J, Cámara A, Fernández M, Fortea J, Buongiorno M, Molinuevo JL, Bargalló N, Martí MJ (2015) Correlates of cerebrospinal fluid levels of oligomeric- and total-α-synuclein in premotor, motor and dementia stages of Parkinson’s disease. J Neurol 262(2):294–306
Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C (2014) Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet (london, England) 383(9916):533–540
Daher JPL, Abdelmotilib HA, Hu X, Volpicelli-Daley LA, Moehle MS, Fraser KB, Needle E, Chen Y, Steyn SJ, Galatsis P, Hirst WD, West AB (2015) Leucine-rich repeat kinase 2 (LRRK2) pharmacological inhibition abates α-synuclein gene-induced neurodegeneration. J Biol Chem 290(32):19433–19444
Davis RL, Wong SL, Carling PJ, Payne T, Sue CM, Bandmann O (2020) Serum FGF-21, GDF-15, and blood mtDNA copy number are not biomarkers of Parkinson disease. Neurol Clin Pract 10(1):40–46
Day JO, Mullin S (2021) The genetics of Parkinson’ disease and implications for clinical practice. Genes 12(7):1006
de Cock VC, Dodet P, Leu-Semenescu S, Aerts C, Castelnovo G, Abril B, Drapier S, Olivet H, Corbillé A-G, Leclair-Visonneau L, Sallansonnet-Froment M, Lebouteux M, Anheim M, Ruppert E, Vitello N, Eusebio A, Lambert I, Marques A, Fantini ML, Devos D, Monaca C, Benard-Serre N, Lacombe S, Vidailhet M, Arnulf I, Doulazmi M, Roze E (2022) Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson’s disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol 21(5):428–437
Dehestani M, Liu H, Gasser T (2021) Polygenic risk scores contribute to personalized medicine of Parkinson’s disease. J Person Med 11(10):1030
Dehestani M, Liu H, Sreelatha AAK, Schulte C, Bansal V, Gasser T (2022) Mitochondrial and autophagy-lysosomal pathway polygenic risk scores predict Parkinson’s disease. Mol Cell Neurosci 121:103751
DeKarske D, Alva G, Aldred JL, Coate B, Cantillon M, Jacobi L, Nunez R, Norton JC, Abler V (2020) An open-label, 8-week study of safety and efficacy of pimavanserin treatment in adults with Parkinson’s disease and depression. J Parkinsons Dis 10(4):1751–1761
Del Din S, Kirk C, Yarnall AJ, Rochester L, Hausdorff JM (2021) Body-worn sensors for remote monitoring of Parkinson’s disease motor symptoms: vision, state of the art, and challenges ahead. J Parkinsons Dis 11(s1):S35–S47
den Heijer JM, Kruithof AC, van Amerongen G, de Kam ML, Thijssen E, Grievink HW, Moerland M, Walker M, Been K, Skerlj R, Justman C, Dudgeon L, Lansbury P, Cullen VC, Hilt DC, Groeneveld GJ (2021) A randomized single and multiple ascending dose study in healthy volunteers of LTI-291, a centrally penetrant glucocerebrosidase activator. Br J Clin Pharmacol 87(9):3561–3573
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider G-H, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355(9):896–908
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52(6):1830–1836
Di Luca DG, Reyes NGD, Fox SH (2022) Newly approved and investigational drugs for motor symptom control in Parkinson’s disease. Drugs 82(10):1027–1053
Dijk JM, Espay AJ, Katzenschlager R, de Bie RMA (2020) The choice between advanced therapies for Parkinson’s disease patients: why, what, and when? J Parkinsons Dis 10(s1):S65–S73
Do J, McKinney C, Sharma P, Sidransky E (2019) Glucocerebrosidase and its relevance to Parkinson disease. Mol Neurodegener 14(1):36
Dorsey ER, Bloem BR (2018) The parkinson pandemic-A call to action. JAMA Neurol 75(1):9–10
Dorsey ER, Elbaz A, Nichols E, Abbasi N, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi J-YJ, Collado-Mateo D, Dahodwala N, Do HP, Edessa D, Endres M, Fereshtehnejad S-M, Foreman KJ, Gankpe FG, Gupta R, Hamidi S, Hankey GJ, Hay SI, Hegazy MI, Hibstu DT, Kasaeian A, Khader Y, Khalil I, Khang Y-H, Kim YJ, Kokubo Y, Logroscino G, Massano J, Mohamed Ibrahim N, Mohammed MA, Mohammadi A, Moradi-Lakeh M, Naghavi M, Nguyen BT, Nirayo YL, Ogbo FA, Owolabi MO, Pereira DM, Postma MJ, Qorbani M, Rahman MA, Roba KT, Safari H, Safiri S, Satpathy M, Sawhney M, Shafieesabet A, Shiferaw MS, Smith M, Szoeke CEI, Tabarés-Seisdedos R, Truong NT, Ukwaja KN, Venketasubramanian N, Villafaina S, Weldegwergs KG, Westerman R, Wijeratne T, Winkler AS, Xuan BT, Yonemoto N, Feigin VL, Vos T, Murray CJL (2018) Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 17(11):939–953
Dossi G, Squarcina L, Rango M (2019) In vivo mitochondrial function in idiopathic and genetic Parkinson’s disease. Metabolites 10(1):19
Eisenberg HM, Krishna V, Elias WJ, Cosgrove GR, Gandhi D, Aldrich CE, Fishman PS (2020) MR-guided focused ultrasound pallidotomy for Parkinson’s disease: safety and feasibility. J Neurosurg 135(3):1–7
Ernst M, Folkerts A-K, Gollan R, Lieker E, Caro-Valenzuela J, Adams A, Cryns N, Monsef I, Dresen A, Roheger M, Eggers C, Skoetz N, Kalbe E (2023) Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database Syst Rev 1(1):CD013856
Espay AJ, Brundin P, Lang AE (2017a) Precision medicine for disease modification in Parkinson disease. Nat Rev Neurol 13(2):119–126
Espay AJ, Schwarzschild MA, Tanner CM, Fernandez HH, Simon DK, Leverenz JB, Merola A, Chen-Plotkin A, Brundin P, Kauffman MA, Erro R, Kieburtz K, Woo D, Macklin EA, Standaert DG, Lang AE (2017b) Biomarker-driven phenotyping in Parkinson’s disease: a translational missing link in disease-modifying clinical trials. Mov Disord 32(3):319–324
Espay AJ, Hausdorff JM, Sánchez-Ferro Á, Klucken J, Merola A, Bonato P, Paul SS, Horak FB, Vizcarra JA, Mestre TA, Reilmann R, Nieuwboer A, Dorsey ER, Rochester L, Bloem BR, Maetzler W (2019) A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord 34(5):657–663
Eusebi P, Giannandrea D, Biscetti L, Abraha I, Chiasserini D, Orso M, Calabresi P, Parnetti L (2017) Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 32(10):1389–1400
Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64(3):343–349
Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, Joachim C, Esiri M, Evetts SG, Rolinski M, Baig F, Ruffmann C, Wade-Martins R, Hu MTM, Parkkinen L, Green AJE (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3(10):812–818
Farbman ES, Waters CH, LeWitt PA, Rudzińska M, Klingler M, Lee A, Qian J, Oh C, Hauser RA (2020) A 12-month, dose-level blinded safety and efficacy study of levodopa inhalation powder (CVT-301, Inbrija) in patients with Parkinson’s disease. Parkinsonism Relat Disord 81:144–150
Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L (2017) Falls in Parkinson’s disease: a complex and evolving picture. Mov Disord 32(11):1524–1536
Fernández-Santiago R, Sharma M (2022) What have we learned from genome-wide association studies (GWAS) in Parkinson’s disease? Ageing Res Rev 79:101648
Fox SH, Katzenschlager R, Lim S-Y, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C (2018) International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33(8):1248–1266
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344(10):710–719
Gallay MN, Moser D, Rossi F, Magara AE, Strasser M, Bühler R, Kowalski M, Pourtehrani P, Dragalina C, Federau C, Jeanmonod D (2019) MRgFUS pallidothalamic tractotomy for chronic therapy-resistant Parkinson’s disease in 51 consecutive patients: single center experience. Front Surg 6:76
Garrido A, Fairfoul G, Tolosa ES, Martí MJ, Green A (2019) α-synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease. Ann Clin Transl Neurol 6(6):1024–1032
Giladi N, Gurevich T, Djaldetti R, Adar L, Case R, Leibman-Barak S, Sasson N, Caraco Y (2021) ND0612 (levodopa/carbidopa for subcutaneous infusion) in patients with Parkinson’s disease and motor response fluctuations: a randomized, placebo-controlled phase 2 study. Parkinsonism Relat Disord 91:139–145
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9(1):13–24
Gohsler B (2021) Amylyx Pharmaceuticals Announces Results from PEGASUS Trial of AMX0035 in Alzheimer’s Disease at the Clinical Trials on Alzheimer’s Disease (CTAD) Conference
Golfrè Andreasi N, Cilia R, Romito LM, Bonvegna S, Straccia G, Elia AE, Novelli A, Messina G, Tringali G, Levi V, Devigili G, Rinaldo S, Gasparini V, Grisoli M, Stanziano M, Ghielmetti F, Prioni S, Bocchi E, Amami P, Piacentini SHMJ, Ciceri EFM, Bruzzone MG, Eleopra R (2022) Magnetic resonance-guided focused ultrasound thalamotomy may spare dopaminergic therapy in early-stage tremor-dominant Parkinson’s disease: a pilot study. Mov Disord 37(11):2289–2295
Gonzalez MC, Ashton NJ, Gomes BF, Tovar-Rios DA, Blanc F, Karikari TK, Mollenhauer B, Pilotto A, Lemstra A, Paquet C, Abdelnour C, Kramberger MG, Bonanni L, Vandenberghe R, Hye A, Blennow K, Zetterberg H, Aarsland D (2022) Association of plasma p-tau181 and p-tau231 concentrations with cognitive decline in patients with probable dementia with lewy bodies. JAMA Neurol 79(1):32–37
Greenland JC, Cutting E, Kadyan S, Bond S, Chhabra A, Williams-Gray CH (2020) Azathioprine immunosuppression and disease modification in Parkinson’s disease (AZA-PD): a randomised double-blind placebo-controlled phase II trial protocol. BMJ Open 10(11):e040527
Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, Campbell KJ, Safar J, Galasko D, Caughey B (2018) Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 6(1):7
Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Håkan W, Decraemer H, Någga K, Minthon L, Londos E, Vanmechelen E, Holmberg B, Zetterberg H, Blennow K, Hansson O (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69(11):1445–1452
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88(10):930–937
Harms AS, Ferreira SA, Romero-Ramos M (2021) Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol 141(4):527–545
Hassan A, Mari Z, Gatto EM, Cardozo A, Youn J, Okubadejo N, Bajwa JA, Shalash A, Fujioka S, Aldaajani Z, Cubo E (2020) global survey on telemedicine utilization for movement disorders during the COVID-19 pandemic. Mov Disord 35(10):1701–1711
Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, Ondo W, O’Connell M, Gupta S (2013) Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 12(4):346–356
Hauser RA, Espay AJ, LeWitt P, Ellenbogen A, Isaacson S, Pahwa R, Stocchi F, Visser H, D’Souza R (2022) A Phase 3 Trial of IPX203 vs CD-LD IR in Parkinson’s Disease Patients with Motor Fluctuations (RISE-PD). AAN Annual Meeting (Abstract 001225)
Herbert MK, Aerts MB, Beenes M, Norgren N, Esselink RAJ, Bloem BR, Kuiperij HB, Verbeek MM (2015) CSF neurofilament light chain but not FLT3 ligand discriminates Parkinsonian disorders. Front Neurol 6:91
Herring WJ, Ceesay P, Snyder E, Bliwise D, Budd K, Hutzelmann J, Stevens J, Lines C, Michelson D (2020) Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimer Dement 16(3):541–551
Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S (2015) Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci 128(2):232–238
Höllerhage M, Wolff A, Chakroun T, Evsyukov V, Duan L, Chua OW-H, Tang Q, Koeglsperger T, Höglinger GU (2022) Binding stability of antibody-α-synuclein complexes predicts the protective efficacy of anti-α-synuclein antibodies. Mol Neurobiol 59(7):3980–3995
Isaacson SH, Boroojerdi B, Waln O, McGraw M, Kreitzman DL, Klos K, Revilla FJ, Heldman D, Phillips M, Terricabras D, Markowitz M, Woltering F, Carson S, Truong D (2019) Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson’s disease starting transdermal rotigotine patch: a pilot study. Parkinsonism Relat Disord 64:132–137
Isaacson SH, Ballard CG, Kreitzman DL, Coate B, Norton JC, Fernandez HH, Ilic TV, Azulay J-P, Ferreira JJ, Abler V, Stankovic S (2021) Efficacy results of pimavanserin from a multi-center, open-label extension study in Parkinson’s disease psychosis patients. Parkinsonism Relat Disord 87:25–31
Jankovic J, Goodman I, Safirstein B, Marmon TK, Schenk DB, Koller M, Zago W, Ness DK, Griffith SG, Grundman M, Soto J, Ostrowitzki S, Boess FG, Martin-Facklam M, Quinn JF, Isaacson SH, Omidvar O, Ellenbogen A, Kinney GG (2018) Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α-synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol 75(10):1206–1214
Jellinger KA (2012) Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord 27(1):8–30
Jennings D, Siderowf A, Stern M, Seibyl J, Eberly S, Oakes D, Marek K (2014) Imaging prodromal Parkinson disease: the Parkinson associated risk syndrome study. Neurology 83(19):1739–1746
Jennings D, Huntwork-Rodriguez S, Vissers MFJM, Daryani VM, Diaz D, Goo MS, Chen JJ, Maciuca R, Fraser K, Mabrouk OS, van de Wetering de Rooij J, Heuberger JAAC, Groeneveld GJ, Borin MT, Cruz-Herranz A, Graham D, Scearce-Levie K, de Vicente J, Henry AG, Chin P, Ho C, Troyer MD (2023) LRRK2 inhibition by BIIB122 in healthy participants and patients with Parkinson’s disease. Mov Disord 38(3):386–398
Jiang C, Hopfner F, Katsikoudi A, Hein R, Catli C, Evetts S, Huang Y, Wang H, Ryder JW, Kuhlenbaeumer G, Deuschl G, Padovani A, Berg D, Borroni B, Hu MT, Davis JJ, Tofaris GK (2020) Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry 91(7):720–729
Jiang C, Hopfner F, Berg D, Hu MT, Pilotto A, Borroni B, Davis JJ, Tofaris GK (2021) Validation of α-synuclein in L1CAM-immunocaptured exosomes as a biomarker for the stratification of parkinsonian syndromes. Mov Disord 36(11):2663–2669
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Staines H, Lees A (2018) Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol 17(9):749–759
Kluge A, Bunk J, Schaeffer E, Drobny A, Xiang W, Knacke H, Bub S, Lückstädt W, Arnold P, Lucius R, Berg D, Zunke F (2022) Detection of neuron-derived pathological α-synuclein in blood. Brain 145(9):3058–3071
Knippenberg E, Verbrugghe J, Lamers I, Palmaers S, Timmermans A, Spooren A (2017) Markerless motion capture systems as training device in neurological rehabilitation: a systematic review of their use, application, target population and efficacy. J Neuroeng Rehabil 14(1):61
Krishna V, Fishman PS, Eisenberg HM, Kaplitt M, Baltuch G, Chang JW, Chang W-C, Martinez Fernandez R, Del Alamo M, Halpern CH, Ghanouni P, Eleopra R, Cosgrove R, Guridi J, Gwinn R, Khemani P, Lozano AM, McDannold N, Fasano A, Constantinescu M, Schlesinger I, Dalvi A, Elias WJ (2023) Trial of globus pallidus focused ultrasound ablation in Parkinson’s disease. N Engl J Med 388(8):683–693
Kuhner A, Wiesmeier IK, Cenciarini M, Maier TL, Kammermeier S, Coenen VA, Burgard W, Maurer C (2019) Motion biomarkers showing maximum contrast between healthy subjects and Parkinson’s disease patients treated with deep brain stimulation of the subthalamic nucleus. Pilot Study Front Neurosci 13:1450
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VGF, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59(3):459–466
Lang AE, Siderowf AD, Macklin EA, Poewe W, Brooks DJ, Fernandez HH, Rascol O, Giladi N, Stocchi F, Tanner CM, Postuma RB, Simon DK, Tolosa E, Mollenhauer B, Cedarbaum JM, Fraser K, Xiao J, Evans KC, Graham DL, Sapir I, Inra J, Hutchison RM, Yang M, Fox T, Budd Haeberlein S, Dam T (2022) Trial of cinpanemab in early Parkinson’s disease. N Engl J Med 387(5):408–420
Lee JW, Song YS, Kim H, Ku BD, Lee WW (2021) Patients with scans without evidence of dopaminergic deficit (SWEDD) do not have early Parkinson’s disease: analysis of the PPMI data. PLoS One 16(2):e0246881
Lemke MR, Fuchs G, Gemende I, Herting B, Oehlwein C, Reichmann H, Rieke J, Volkmann J (2004) Depression and Parkinson’s disease. J Neurol 251(Suppl 6):Vi/24-7
Lerche S, Schulte C, Wurster I, Machetanz G, Roeben B, Zimmermann M, Deuschle C, Hauser A-K, Böhringer J, Krägeloh-Mann I, Waniek K, Lachmann I, Petterson X-MT, Chiang R, Park H, Wang B, Liepelt-Scarfone I, Maetzler W, Galasko D, Scherzer CR, Gasser T, Mielke MM, Hutten SJ, Mollenhauer B, Sardi SP, Berg D, Brockmann K (2021) The mutation matters: CSF profiles of GCase, sphingolipids, α-synuclein in PDGBA. Mov Disord 36(5):1216–1228
Levin J, Sing N, Melbourne S, Morgan A, Mariner C, Spillantini MG, Wegrzynowicz M, Dalley JW, Langer S, Ryazanov S, Leonov A, Griesinger C, Schmidt F, Weckbecker D, Prager K, Matthias T, Giese A (2022) Safety, tolerability and pharmacokinetics of the oligomer modulator anle138b with exposure levels sufficient for therapeutic efficacy in a murine Parkinson model: a randomised, double-blind, placebo-controlled phase 1a trial. EBioMedicine 80:104021
LeWitt PA, Hauser RA, Pahwa R, Isaacson SH, Fernandez HH, Lew M, Saint-Hilaire M, Pourcher E, Lopez-Manzanares L, Waters C, Rudzínska M, Sedkov A, Batycky R, Oh C (2019) Safety and efficacy of CVT-301 (levodopa inhalation powder) on motor function during off periods in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Neurol 18(2):145–154
LeWitt PA, Stocchi F, Arkadir D, Caraco Y, Adar L, Perlstein I, Case R, Giladi N (2022) The pharmacokinetics of continuous subcutaneous levodopa/carbidopa infusion: findings from the ND0612 clinical development program. Front Neurol 13:1036068
Lhermitte J, Kraus WM, McAlpine D (1924) Original papers: on the occurrence of abnormal deposits of iron in the brain in Parkinsonism with special reference to its localisation. J Neurol Psychopathol 5(19):195–208
Li C, Xue L, Liu Y, Yang Z, Chi S, Xie A (2020) Zonisamide for the treatment of parkinson disease: a current update. Front Neurosci 14:574652
Lin C-H, Yang S-Y, Horng H-E, Yang C-C, Chieh J-J, Chen H-H, Liu B-H, Chiu M-J (2018) Plasma biomarkers differentiate Parkinson’s disease from atypical Parkinsonism syndromes. Front Aging Neurosci 10:123
Lin C-H, Li C-H, Yang K-C, Lin F-J, Wu C-C, Chieh J-J, Chiu M-J (2019) Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 93(11):e1104–e1111
Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D (2011) MDS task force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord 26(10):1814–1824
Lucio M, Willkommen D, Schroeter M, Sigaroudi A, Schmitt-Kopplin P, Michalke B (2019) Integrative metabolomic and metallomic analysis in a case-control cohort with Parkinson’s disease. Front Aging Neurosci 11:331
Maass F, Michalke B, Leha A, Boerger M, Zerr I, Koch J-C, Tönges L, Bähr M, Lingor P (2018) Elemental fingerprint as a cerebrospinal fluid biomarker for the diagnosis of Parkinson’s disease. J Neurochem 145(4):342–351
Maass F, Michalke B, Willkommen D, Canaslan S, Schmitz M, Bähr M, Zerr I, Lingor P (2021) Cerebrospinal fluid iron-ferritin ratio as a potential progression marker for Parkinson’s disease. Mov Disord 36(12):2967–2969
Majbour NK, Vaikath NN, Eusebi P, Chiasserini D, Ardah M, Varghese S, Haque ME, Tokuda T, Auinger P, Calabresi P, Parnetti L, El-Agnaf OMA (2016) Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov Disord 31(10):1535–1542
Majbour N, Aasly J, Abdi I, Ghanem S, Erskine D, van de Berg W, El-Agnaf O (2022) Disease-associated α-synuclein aggregates as biomarkers of parkinson disease clinical stage. Neurology 99(21):e2417–e2427
Manne S, Kondru N, Hepker M, Jin H, Anantharam V, Lewis M, Huang X, Kanthasamy A, Kanthasamy AG (2019) Ultrasensitive detection of aggregated α-synuclein in glial cells, human cerebrospinal fluid, and brain tissue using the RT-QuIC assay: new high-throughput neuroimmune biomarker assay for Parkinsonian disorders. J Neuroimmune Pharmacol 14(3):423–435
Manzoni C, Mamais A, Dihanich S, Abeti R, Soutar MPM, Plun-Favreau H, Giunti P, Tooze SA, Bandopadhyay R, Lewis PA (2013) Inhibition of LRRK2 kinase activity stimulates macroautophagy. Biochem Biophys Acta 12:2900–2910
Mariani S, Ventriglia M, Simonelli I, Donno S, Bucossi S, Vernieri F, Melgari J-M, Pasqualetti P, Rossini PM, Squitti R (2013) Fe and Cu do not differ in Parkinson’s disease: a replication study plus meta-analysis. Neurobiol Aging 34(2):632–633
Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ (2018) Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol 17(6):559–568
Marques TM, van Rumund A, Oeckl P, Kuiperij HB, Esselink RAJ, Bloem BR, Otto M, Verbeek MM (2019) Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92(13):e1479–e1486
Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, Del Álamo M, Shah BB, Hernández-Fernández F, Pineda-Pardo JA, Monje MHG, Fernández-Rodríguez B, Sperling SA, Mata-Marín D, Guida P, Alonso-Frech F, Obeso I, Gasca-Salas C, Vela-Desojo L, Elias WJ, Obeso JA (2020) Randomized trial of focused ultrasound subthalamotomy for Parkinson’s disease. N Engl J Med 383(26):2501–2513
Martínez-Fernández R, Matarazzo M, Máñez-Miró JU, Obeso JA (2021) The role of focused ultrasound in the management of movement disorders: insights after 5 years of experience. Mov Disord Clin Pract 8(5):681–687
Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D (2005) Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46(6):857–868
Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D (2011) Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One 6(4):e19338
McFarthing K, Rafaloff G, Baptista M, Mursaleen L, Fuest R, Wyse RK, Stott SRW (2022) Parkinson’s Disease drug therapies in the clinical trial pipeline: 2022 update. J Parkinsons Dis 12(4):1073–1082
Medeiros MS, Schumacher-Schuh A, Cardoso AM, Bochi GV, Baldissarelli J, Kegler A, Santana D, Chaves CMMBS, Schetinger MRC, Moresco RN, Rieder CRM, Fighera MR (2016) Iron and oxidative stress in Parkinson’s disease: an observational study of injury biomarkers. PLoS One 11(1):e0146129
Mestre TA, Fereshtehnejad S-M, Berg D, Bohnen NI, Dujardin K, Erro R, Espay AJ, Halliday G, van Hilten JJ, Hu MT, Jeon B, Klein C, Leentjens AFG, Marinus J, Mollenhauer B, Postuma R, Rajalingam R, Rodríguez-Violante M, Simuni T, Surmeier DJ, Weintraub D, McDermott MP, Lawton M, Marras C (2021) Parkinson’s disease subtypes: critical appraisal and recommendations. J Parkinsons Dis 11(2):395–404
Miglis MG, Adler CH, Antelmi E, Arnaldi D, Baldelli L, Boeve BF, Cesari M, Dall’Antonia I, Diederich NJ, Doppler K, Dušek P, Ferri R, Gagnon J-F, Gan-Or Z, Hermann W, Högl B, Hu MT, Iranzo A, Janzen A, Kuzkina A, Lee J-Y, Leenders KL, Lewis SJG, Liguori C, Liu J, Lo C, Ehgoetz Martens KA, Nepozitek J, Plazzi G, Provini F, Puligheddu M, Rolinski M, Rusz J, Stefani A, Summers RLS, Yoo D, Zitser J, Oertel WH (2021) Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol 20(8):671–684
Miller T, Cudkowicz M, Shaw PJ, Andersen PM, Atassi N, Bucelli RC, Genge A, Glass J, Ladha S, Ludolph AL, Maragakis NJ, McDermott CJ, Pestronk A, Ravits J, Salachas F, Trudell R, van Damme P, Zinman L, Bennett CF, Lane R, Sandrock A, Runz H, Graham D, Houshyar H, McCampbell A, Nestorov I, Chang I, McNeill M, Fanning L, Fradette S, Ferguson TA (2020) Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med 383(2):109–119
Miller TM, Cudkowicz ME, Genge A, Shaw PJ, Sobue G, Bucelli RC, Chiò A, van Damme P, Ludolph AC, Glass JD, Andrews JA, Babu S, Benatar M, McDermott CJ, Cochrane T, Chary S, Chew S, Zhu H, Wu F, Nestorov I, Graham D, Sun P, McNeill M, Fanning L, Ferguson TA, Fradette S (2022) Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med 387(12):1099–1110
Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Ng J, Schulz-Schaeffer W, Kretzschmar HA, McLean PJ, Trenkwalder C, Sarracino DA, Vonsattel J-P, Locascio JJ, El-Agnaf OMA, Schlossmacher MG (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 213(2):315–325
Mollenhauer B, Caspell-Garcia CJ, Coffey CS, Taylor P, Shaw LM, Trojanowski JQ, Singleton A, Frasier M, Marek K, Galasko D (2017) Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 89(19):1959–1969
Moosa S, Martínez-Fernández R, Elias WJ, Del Alamo M, Eisenberg HM, Fishman PS (2019) The role of high-intensity focused ultrasound as a symptomatic treatment for Parkinson’s disease. Mov Disord 34(9):1243–1251
Mortiboys H, Aasly J, Bandmann O (2013) Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain 136(Pt 10):3038–3050
Müller-Nedebock AC, Meldau S, Lombard C, Abrahams S, van der Westhuizen FH, Bardien S (2022) Increased blood-derived mitochondrial DNA copy number in African ancestry individuals with Parkinson’s disease. Parkinsonism Relat Disord 101:1–5
Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D (2019) Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson’s disease. Trends Neurosci 42(2):140–149
NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators (2015) Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol 14(8):795–803
Ohrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, Zetterberg H (2009) Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett 450(3):332–335
Okun MS (2012) Deep-brain stimulation for Parkinson’s disease. N Engl J Med 367(16):1529–1538
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54(3):403–414
Olanow CW, Kordower JH, Lang AE, Obeso JA (2009) Dopaminergic transplantation for Parkinson’s disease: current status and future prospects. Ann Neurol 66(5):591–596
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13(2):141–149
Olanow CW, Bartus RT, Volpicelli-Daley LA, Kordower JH (2015) Trophic factors for Parkinson’s disease: to live or let die. Mov Disord 30(13):1715–1724
Olanow CW, Kieburtz K, Leinonen M, Elmer L, Giladi N, Hauser RA, Klepiskaya OS, Kreitzman DL, Lew MF, Russell DS, Kadosh S, Litman P, Friedman H, Linvah N, The P B Study Group F (2017) A randomized trial of a low-dose rasagiline and pramipexole combination (P2B001) in early Parkinson’s disease. Mov Disord 32(5):783–789
Olanow CW, Factor SA, Espay AJ, Hauser RA, Shill HA, Isaacson S, Pahwa R, Leinonen M, Bhargava P, Sciarappa K, Navia B, Blum D (2020) Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol 19(2):135–144
Olanow CW, Espay AJ, Stocchi F, Ellenbogen AL, Leinonen M, Adar L, Case RJ, Orenbach SF, Yardeni T, Oren S, Poewe W (2021) Continuous subcutaneous levodopa delivery for Parkinson’s disease: a randomized study. J Parkinsons Dis 11(1):177–186
Pagano G, Boess FG, Taylor KI, Ricci B, Mollenhauer B, Poewe W, Boulay A, Anzures-Cabrera J, Vogt A, Marchesi M, Post A, Nikolcheva T, Kinney GG, Zago WM, Ness DK, Svoboda H, Britschgi M, Ostrowitzki S, Simuni T, Marek K, Koller M, Sevigny J, Doody R, Fontoura P, Umbricht D, Bonni A (2021) A phase II study to evaluate the safety and efficacy of prasinezumab in early Parkinson’s disease (PASADENA): rationale, design, and baseline data. Front Neurol 12:705407
Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, Postuma RB, Pavese N, Stocchi F, Azulay J-P, Mollenhauer B, López-Manzanares L, Russell DS, Boyd JT, Nicholas AP, Luquin MR, Hauser RA, Gasser T, Poewe W, Ricci B, Boulay A, Vogt A, Boess FG, Dukart J, D’Urso G, Finch R, Zanigni S, Monnet A, Pross N, Hahn A, Svoboda H, Britschgi M, Lipsmeier F, Volkova-Volkmar E, Lindemann M, Dziadek S, Holiga Š, Rukina D, Kustermann T, Kerchner GA, Fontoura P, Umbricht D, Doody R, Nikolcheva T, Bonni A (2022) Trial of prasinezumab in early-stage Parkinson’s disease. N Engl J Med 387(5):421–432
Paganoni S, Watkins C, Cawson M, Hendrix S, Dickson SP, Knowlton N, Timmons J, Manuel M, Cudkowicz M (2022) Survival analyses from the CENTAUR trial in amyotrophic lateral sclerosis: Evaluating the impact of treatment crossover on outcomes. Muscle Nerve 66(2):136–141
Pagonabarraga J, Pérez-González R, Bejr-Kasem H, Marín-Lahoz J, Horta-Barba A, Martinez-Horta S, Aracil-Bolaños I, Sampedro F, Campolongo A, Rivas E, Puig-Davi A, Ruiz-Barrios I, Pérez-Pérez J, Pascual-Sedano B, Kulisevsky J (2022) Dissociable contribution of plasma NfL and p-tau181 to cognitive impairment in Parkinson’s disease. Parkinsonism Relat Disord 105:132–138
Papagiannakis N, Xilouri M, Koros C, Stamelou M, Antonelou R, Maniati M, Papadimitriou D, Moraitou M, Michelakakis H, Stefanis L (2015) Lysosomal alterations in peripheral blood mononuclear cells of Parkinson’s disease patients. Mov Disord 30(13):1830–1834
Parkinson Study Group (2002) Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287(13):1653–1661
Paul KC, Schulz J, Bronstein JM, Lill CM, Ritz BR (2018) Association of polygenic risk score with cognitive decline and motor progression in Parkinson disease. JAMA Neurol 75(3):360–366
Perra D, Bongianni M, Novi G, Janes F, Bessi V, Capaldi S, Sacchetto L, Tagliapietra M, Schenone G, Morbelli S, Fiorini M, Cattaruzza T, Mazzon G, Orrù CD, Catalan M, Polverino P, Bernardini A, Pellitteri G, Valente M, Bertolotti C, Nacmias B, Maggiore G, Cavallaro T, Manganotti P, Gigli G, Monaco S, Nobili F, Zanusso G (2021) Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun 3(2):fcab045
Peterschmitt MJ, Saiki H, Hatano T, Gasser T, Isaacson SH, Gaemers SJM, Minini P, Saubadu S, Sharma J, Walbillic S, Alcalay RN, Cutter G, Hattori N, Höglinger GU, Marek K, Schapira AHV, Scherzer CR, Simuni T, Giladi N, Sardi SP, Fischer TZ (2022) Safety, pharmacokinetics, and pharmacodynamics of oral venglustat in patients with Parkinson’s disease and a GBA mutation: results from part 1 of the randomized, double-blinded, placebo-controlled MOVES-PD trial. J Parkinsons Dis 12(2):557–570
Pihlstrøm L, Fan CC, Frei O, Tan M, Karunamuni RA, Blauwendraat C, Bandres-Ciga S, Gan-Or Z, Grosset DG, Dale AM, Seibert TM, Andreassen OA (2022) Genetic stratification of age-dependent Parkinson’s disease risk by polygenic hazard score. Mov Disord 37(1):62–69
Poewe W, Stocchi F, Arkadir D, Ebersbach G, Ellenbogen AL, Giladi N, Isaacson SH, Kieburtz K, LeWitt P, Olanow CW, Simuni T, Thomas A, Zlotogorski A, Adar L, Case R, Oren S, Fuchs Orenbach S, Rosenfeld O, Sasson N, Yardeni T, Espay AJ (2021) Subcutaneous levodopa infusion for Parkinson’s disease: 1-year data from the open-label BeyoND study. Mov Disord 36(11):2687–2692
Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE, Bain P, Molloy S, Piccini P (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Investig 124(3):1340–1349
Poly TN, Islam MMR, Yang H-C, Li Y-CJ (2019) Non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease in the elderly population: a meta-analysis. Eur J Clin Pharmacol 75(1):99–108
Powers R, Etezadi-Amoli M, Arnold EM, Kianian S, Mance I, Gibiansky M, Trietsch D, Alvarado AS, Kretlow JD, Herrington TM, Brillman S, Huang N, Lin PT, Pham HA, Ullal AV (2021) Smartwatch inertial sensors continuously monitor real-world motor fluctuations in Parkinson’s disease. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abd7865
Prasuhn J, Davis RL, Kumar KR (2020) Targeting Mitochondrial Impairment in Parkinson’s disease: challenges and opportunities. Front Cell Dev Biol 8:615461
Price DL, Koike MA, Khan A, Wrasidlo W, Rockenstein E, Masliah E, Bonhaus D (2018) The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson’s disease. Sci Rep 8(1):16165
Pyle A, Anugrha H, Kurzawa-Akanbi M, Yarnall A, Burn D, Hudson G (2016) Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiol Aging 38:216.e7-216.e10
Qin X-Y, Zhang S-P, Cao C, Loh YP, Cheng Y (2016) Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol 73(11):1316–1324
Rabinovici GD (2021) Controversy and progress in Alzheimer’s disease—FDA approval of aducanumab. N Engl J Med 385(9):771–774
Rawner E (2021) A randomized, double-blind, placebo-controlled trial assessing the safety and tolerability of GRF6021 in subjects with Parkinson’s disease and cognitive impairment. International Conference on Alzheimer’s and Parkinson’s Disease
Rein-Hedin E, Sjöberg F, Waters S, Sonesson C, Waters N, Huss F, Tedroff J (2021) First-in-human study to assess the safety, tolerability, and pharmacokinetics of pirepemat, a cortical enhancer, in healthy volunteers. Clin Pharmacol Drug Dev 10(12):1485–1494
Riesenberg R, Werth J, Zhang Y, Duvvuri S, Gray D (2020) PF-06649751 efficacy and safety in early Parkinson’s disease: a randomized, placebo-controlled trial. Ther Adv Neurol Disord 13:1756286420911296
Rocco MT, Akhter AS, Ehrlich DJ, Scott GC, Lungu C, Munjal V, Aquino A, Lonser RR, Fiandaca MS, Hallett M, Heiss JD, Bankiewicz KS (2022) Long-term safety of MRI-guided administration of AAV2-GDNF and gadoteridol in the putamen of individuals with Parkinson’s disease. Mol Ther 30(12):3632–3638
Rosebraugh M, Liu W, Neenan M, Facheris MF (2021a) Foslevodopa/foscarbidopa is well tolerated and maintains stable levodopa and carbidopa exposure following subcutaneous infusion. J Parkinsons Dis 11(4):1695–1702
Rosebraugh M, Voight EA, Moussa EM, Jameel F, Lou X, Zhang GGZ, Mayer PT, Stolarik D, Carr RA, Enright BP, Liu W, Facheris MF, Kym PR (2021b) Foslevodopa/foscarbidopa: a new subcutaneous treatment for Parkinson’s disease. Ann Neurol 90(1):52–61
Rosen A, Zeger SL (2019) Precision medicine: discovering clinically relevant and mechanistically anchored disease subgroups at scale. J Clin Investig 129(3):944–945
Rossi M, Candelise N, Baiardi S, Capellari S, Giannini G, Orrù CD, Antelmi E, Mammana A, Hughson AG, Calandra-Buonaura G, Ladogana A, Plazzi G, Cortelli P, Caughey B, Parchi P (2020) Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol 140(1):49–62
Russo MJ, Orru CD, Concha-Marambio L, Giaisi S, Groveman BR, Farris CM, Holguin B, Hughson AG, Lafontant D-E, Caspell-Garcia C, Coffey CS, Mollon J, Hutten SJ, Merchant K, Heym RG, Soto C, Caughey B, Kang UJ (2021) High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol Commun 9(1):179
Saeed U, Compagnone J, Aviv RI, Strafella AP, Black SE, Lang AE, Masellis M (2017) Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: current and emerging concepts. Transl Neurodegener 6:8
Sala G, Stefanoni G, Arosio A, Riva C, Melchionda L, Saracchi E, Fermi S, Brighina L, Ferrarese C (2014) Reduced expression of the chaperone-mediated autophagy carrier hsc70 protein in lymphomonocytes of patients with Parkinson’s disease. Brain Res 1546:46–52
Schalkamp A-K, Peall KJ, Harrison NA, Sandor C (2022) Wearable devices can identify Parkinson’s disease up to 7 years before clinical diagnosis. medRxiv
Schofield DJ, Irving L, Calo L, Bogstedt A, Rees G, Nuccitelli A, Narwal R, Petrone M, Roberts J, Brown L, Cusdin F, Dosanjh B, Lloyd C, Dobson C, Gurrell I, Fraser G, McFarlane M, Rockenstein E, Spencer B, Masliah E, Spillantini MG, Tan K, Billinton A, Vaughan T, Chessell I, Perkinton MS (2019) Preclinical development of a high affinity α-synuclein antibody, MEDI1341, that can enter the brain, sequester extracellular α-synuclein and attenuate α-synuclein spreading in vivo. Neurobiol Dis 132:104582
Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Hälbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider G-H, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto J-L, Bataille B, Maltête D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Krüger R, Pinsker MO, Amtage F, Régis J-M, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368(7):610–622
Schwarzschild MA, Ascherio A, Casaceli C, Curhan GC, Fitzgerald R, Kamp C, Lungu C, Macklin EA, Marek K, Mozaffarian D, Oakes D, Rudolph A, Shoulson I, Videnovic A, Scott B, Gauger L, Aldred J, Bixby M, Ciccarello J, Gunzler SA, Henchcliffe C, Brodsky M, Keith K, Hauser RA, Goetz C, LeDoux MS, Hinson V, Kumar R, Espay AJ, Jimenez-Shahed J, Hunter C, Christine C, Daley A, Leehey M, de Marcaida JA, Friedman JH, Hung A, Bwala G, Litvan I, Simon DK, Simuni T, Poon C, Schiess MC, Chou K, Park A, Bhatti D, Peterson C, Criswell SR, Rosenthal L, Durphy J, Shill HA, Mehta SH, Ahmed A, Deik AF, Fang JY, Stover N, Zhang L, Dewey RB, Gerald A, Boyd JT, Houston E, Suski V, Mosovsky S, Cloud L, Shah BB, Saint-Hilaire M, James R, Zauber SE, Reich S, Shprecher D, Pahwa R, Langhammer A, LaFaver K, LeWitt PA, Kaminski P, Goudreau J, Russell D, Houghton DJ, Laroche A, Thomas K, McGraw M, Mari Z, Serrano C, Blindauer K, Rabin M, Kurlan R, Morgan JC, Soileau M, Ainslie M, Bodis-Wollner I, Schneider RB, Waters C, Ratel AS, Beck CA, Bolger P, Callahan KF, Crotty GF, Klements D, Kostrzebski M, McMahon GM, Pothier L, Waikar SS, Lang A, Mestre T (2021) Effect of urate-elevating inosine on early Parkinson disease progression: the SURE-PD3 randomized clinical trial. JAMA 326(10):926–939
Senek M, Nielsen EI, Nyholm D (2017) Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord 32(2):283–286
Senek M, Nyholm D, Nielsen EI (2020) Population pharmacokinetics of levodopa gel infusion in Parkinson’s disease: effects of entacapone infusion and genetic polymorphism. Sci Rep 10(1):18057
Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, Weintraub D, Sampaio C (2019) Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 34(2):180–198
Shahaduzzaman M, Nash K, Hudson C, Sharif M, Grimmig B, Lin X, Bai G, Liu H, Ugen KE, Cao C, Bickford PC (2015) Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One 10(2):e0116841
Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, Shaw LM, van Deerlin V, Trojanowski JQ, Clark C (2010) CSF amyloid beta 1–42 predicts cognitive decline in Parkinson disease. Neurology 75(12):1055–1061
Siderowf A, Concha-Marambio L, Lafontant D-E, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LMA, Hutten SJ, Sherer T, Marek K, Soto C (2023) Assessment of heterogeneity among participants in the Parkinson’s progression markers initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol 22(5):407–417
Simuni T, Siderowf A, Lasch S, Coffey CS, Caspell-Garcia C, Jennings D, Tanner CM, Trojanowski JQ, Shaw LM, Seibyl J, Schuff N, Singleton A, Kieburtz K, Toga AW, Mollenhauer B, Galasko D, Chahine LM, Weintraub D, Foroud T, Tosun D, Poston K, Arnedo V, Frasier M, Sherer T, Chowdhury S, Marek K (2018) Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s progression markers initiative cohort. Mov Disord 33(5):771–782
Singleton A, Hardy J (2019) Progress in the genetic analysis of Parkinson’s disease. Hum Mol Genet 28(R2):R215–R218
Sjöberg F, Waters S, Löfberg B, Sonesson C, Waters N, Tedroff J (2021) A first-in-human oral dose study of mesdopetam (IRL790) to assess its safety, tolerability, and pharmacokinetics in healthy male volunteers. Pharmacol Res Perspect 9(3):e00792
Skillbäck T, Farahmand BY, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D, Wimo A, Winblad B, Schott JM, Blennow K, Eriksdotter M, Zetterberg H (2015) Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138(Pt 9):2716–2731
Skrahina V, Gaber H, Vollstedt E-J, Förster TM, Usnich T, Curado F, Brüggemann N, Paul J, Bogdanovic X, Zülbahar S, Olmedillas M, Skobalj S, Ameziane N, Bauer P, Csoti I, Koleva-Alazeh N, Grittner U, Westenberger A, Kasten M, Beetz C, Klein C, Rolfs A (2021) The rostock international Parkinson’s disease (ROPAD) study: protocol and initial findings. Mov Disord 36(4):1005–1010
Smit JW, Basile P, Prato MK, Detalle L, Mathy F-X, Schmidt A, Lalla M, Germani M, Domange C, Biere A-L, Bani M, Carson S, Genius J (2022) Phase 1/1b studies of UCB0599, an oral inhibitor of α-synuclein misfolding, including a randomized study in Parkinson’s disease. Mov Disord 37(10):2045–2056
Smith C, Malek N, Grosset K, Cullen B, Gentleman S, Grosset DG (2019) Neuropathology of dementia in patients with Parkinson’s disease: a systematic review of autopsy studies. J Neurol Neurosurg Psychiatry 90(11):1234–1243
Soileau MJ, Aldred J, Budur K, Fisseha N, Fung VS, Jeong A, Kimber TE, Klos K, Litvan I, O’Neill D, Robieson WZ, Spindler MA, Standaert DG, Talapala S, Vaou EO, Zheng H, Facheris MF, Hauser RA (2022) Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol 21(12):1099–1109
Starkstein SE, Brockman S (2017) Management of depression in Parkinson’s disease: a systematic review. Mov Disord Clin Pract 4(4):470–477
Stefani A, Iranzo A, Holzknecht E, Perra D, Bongianni M, Gaig C, Heim B, Serradell M, Sacchetto L, Garrido A, Capaldi S, Sánchez-Gómez A, Cecchini MP, Mariotto S, Ferrari S, Fiorini M, Schmutzhard J, Cocchiara P, Vilaseca I, Brozzetti L, Monaco S, Jose Marti M, Seppi K, Tolosa E, Santamaria J, Högl B, Poewe W, Zanusso G (2021) Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 144(4):1118–1126
Stefanis L, Emmanouilidou E, Pantazopoulou M, Kirik D, Vekrellis K, Tofaris GK (2019) How is alpha-synuclein cleared from the cell? J Neurochem 150(5):577–590
Stevens KN, Creanor S, Jeffery A, Whone A, Zajicek J, Foggo A, Jones B, Chapman R, Cocking L, Wilks J, Webb D, Carroll C (2022) Evaluation of simvastatin as a disease-modifying treatment for patients with Parkinson disease: a randomized clinical trial. JAMA Neurol 79(12):1232–1241
Stocchi F, Antonini A, Berg D, Bergmans B, Jost W, Katzenschlager R, Kulisevsky J, Odin P, Valldeoriola F, Ray Chaudhuri K (2022) Safinamide in the treatment pathway of Parkinson’s disease: a European Delphi consensus. Npj Parkinsons Dis 8(1):17
Suppa A, Costantini G, Asci F, Di Leo P, Al-Wardat MS, Di Lazzaro G, Scalise S, Pisani A, Saggio G (2022) Voice in Parkinson’s disease: a machine learning study. Front Neurol 13:831428
SynAgile Corporation (2022) SynAgile Corporation Announces Positive Phase 2 Top Line Results for DopaFuse® Levodopa-Carbidopa Delivery System. https://www.globenewswire.com/news-release/2022/11/04/2548477/0/en/SynAgile-Corporation-Announces-Positive-Phase-2-Top-Line-Results-for-DopaFuse-Levodopa-Carbidopa-Delivery-System.html. Accessed 14 Apr 2023
Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V (2022) Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 22(11):657–673
Tariot PN, Cummings JL, Soto-Martin ME, Ballard C, Erten-Lyons D, Sultzer DL, Devanand DP, Weintraub D, McEvoy B, Youakim JM, Stankovic S, Foff EP (2021) Trial of pimavanserin in dementia-related psychosis. N Engl J Med 385(4):309–319
Tarolli CG, Zimmerman GA, Auinger P, McIntosh S, Horowitz RK, Kluger BM, Dorsey ER, Holloway RG (2020) Symptom burden among individuals with Parkinson disease: a national survey. Neurol Clin Pract 10(1):65–72
Tatenhorst L, Eckermann K, Dambeck V, Fonseca-Ornelas L, Walle H, Da Lopes FT, Koch JC, Becker S, Tönges L, Bähr M, Outeiro TF, Zweckstetter M, Lingor P (2016) Fasudil attenuates aggregation of α-synuclein in models of Parkinson’s disease. Acta Neuropathol Commun 4:39
The Parkinson Progression Marker Initiative (PPMI) (2011) The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 95(4):629–635
Titova N, Chaudhuri KR (2017) Personalized medicine in Parkinson’s disease: time to be precise. Mov Disord 32(8):1147–1154
Tofaris GK (2022) Initiation and progression of α-synuclein pathology in Parkinson’s disease. Cell Mol Life Sci 79(4):210
Usnich T, Vollstedt E-J, Schell N, Skrahina V, Bogdanovic X, Gaber H, Förster TM, Heuer A, Koleva-Alazeh N, Csoti I, Basak AN, Ertan S, Genc G, Bauer P, Lohmann K, Grünewald A, Schymanski EL, Trinh J, Schaake S, Berg D, Gruber D, Isaacson SH, Kühn AA, Mollenhauer B, Pedrosa DJ, Reetz K, Sammler EM, Valente EM, Valzania F, Volkmann J, Zittel S, Brüggemann N, Kasten M, Rolfs A, Klein C (2021) LIPAD (LRRK2/luebeck international Parkinson’s Disease) study protocol: deep phenotyping of an international genetic cohort. Front Neurol 12:710572
van Dijk KD, Persichetti E, Chiasserini D, Eusebi P, Beccari T, Calabresi P, Berendse HW, Parnetti L, van de Berg WDJ (2013) Changes in endolysosomal enzyme activities in cerebrospinal fluid of patients with Parkinson’s disease. Mov Disord 28(6):747–754
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T (2023) Lecanemab in early Alzheimer’s disease. N Engl J Med 388(1):9–21
Velseboer DC, de Bie RMA, Wieske L, Evans JR, Mason SL, Foltynie T, Schmand B, de Haan RJ, Post B, Barker RA, Williams-Gray CH (2016) Development and external validation of a prognostic model in newly diagnosed Parkinson disease. Neurology 86(11):986–993
Vijiaratnam N, Simuni T, Bandmann O, Morris HR, Foltynie T (2021) Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol 20(7):559–572
Warmerdam E, Hausdorff JM, Atrsaei A, Zhou Y, Mirelman A, Aminian K, Espay AJ, Hansen C, Evers LJW, Keller A, Lamoth C, Pilotto A, Rochester L, Schmidt G, Bloem BR, Maetzler W (2020) Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol 19(5):462–470
Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, Kordower JH, Alterman R, Lozano AM, Lang AE (2015) Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double-blind, randomized, controlled trial. Ann Neurol 78(2):248–257
Waters S, Sonesson C, Svensson P, Tedroff J, Carta M, Ljung E, Gunnergren J, Edling M, Svanberg B, Fagerberg A, Kullingsjö J, Hjorth S, Waters N (2020) Preclinical pharmacology of 2-(3-fluoro-5-methanesulfonyl-phenoxy)ethyl(Propyl)amine (IRL790), a novel dopamine transmission modulator for the treatment of motor and psychiatric complications in Parkinson disease. J Pharmacol Exp Ther 374(1):113–125
Weiß C, Ziegler A, Becker L-L, Johannsen J, Brennenstuhl H, Schreiber G, Flotats-Bastardas M, Stoltenburg C, Hartmann H, Illsinger S, Denecke J, Pechmann A, Müller-Felber W, Vill K, Blaschek A, Smitka M, van der Stam L, Weiss K, Winter B, Goldhahn K, Plecko B, Horber V, Bernert G, Husain RA, Rauscher C, Trollmann R, Garbade SF, Hahn A, von der Hagen M, Kaindl AM (2022) Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15 kg: an observational cohort study. Lancet Child Adolesc Health 6(1):17–27
Weintraub D, Aarsland D, Biundo R, Dobkin R, Goldman J, Lewis S (2022) Management of psychiatric and cognitive complications in Parkinson’s disease. BMJ (clin Res Ed) 379:e068718
Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K (2010) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9(6):581–591
Williams-Gray CH, Wijeyekoon R, Yarnall AJ, Lawson RA, Breen DP, Evans JR, Cummins GA, Duncan GW, Khoo TK, Burn DJ, Barker RA (2016) Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord 31(7):995–1003
Wong YC, Krainc D (2017) α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23(2):1–13
Woodrow H, Horne MK, Fernando CV, Kotschet KE (2020) A blinded, controlled trial of objective measurement in Parkinson’s disease. Npj Parkinsons Dis 6(1):35
Wrasidlo W, Tsigelny IF, Price DL, Dutta G, Rockenstein E, Schwarz TC, Ledolter K, Bonhaus D, Paulino A, Eleuteri S, Skjevik ÅA, Kouznetsova VL, Spencer B, Desplats P, Gonzalez-Ruelas T, Trejo-Morales M, Overk CR, Winter S, Zhu C, Chesselet M-F, Meier D, Moessler H, Konrat R, Masliah E (2016) A de novo compound targeting α-synuclein improves deficits in models of Parkinson’s disease. Brain J Neurol 139(Pt 12):3217–3236
Xiang W, Rappaport J (2021) XWPharma Announces Positive Results from Phase 1 Clinical Trials of XW10172, in Development as Once-Nightly Therapy for Sleep Disorders in Patients with Neurodegenerative Diseases. https://www.globenewswire.com/news-release/2021/06/14/2246472/0/en/XWPharma-Announces-Positive-Results-from-Phase-1-Clinical-Trials-of-XW10172-in-Development-as-Once-Nightly-Therapy-Therapyfor-Sleep-Disorders-in-Patients-with-Neurodegenerative-Diseases.html. Accessed 14 Apr 2023
Xicoy H, Peñuelas N, Vila M, Laguna A (2019) Autophagic- and lysosomal-related biomarkers for Parkinson’s disease: lights and shadows. Cells 8(11):1317
Xu Y, He Q, Wang M, Gao Y, Liu X, Li D, Xiong B, Wang W (2021) Safety and efficacy of magnetic resonance imaging-guided focused ultrasound neurosurgery for Parkinson’s disease: a systematic review. Neurosurg Rev 44(1):115–127
Yang Y, Shi Y, Schweighauser M, Zhang X, Kotecha A, Murzin AG, Garringer HJ, Cullinane PW, Saito Y, Foroud T, Warner TT, Hasegawa K, Vidal R, Murayama S, Revesz T, Ghetti B, Hasegawa M, Lashley T, Scheres SHW, Goedert M (2022) Structures of α-synuclein filaments from human brains with Lewy pathology. Nature 610(7933):791–795
Yu HJ, Thijssen E, van Brummelen E, van der Plas JL, Radanovic I, Moerland M, Hsieh E, Groeneveld GJ, Dodart J-C (2022) A Randomized first-in-human study with UB-312, a UBITh® α-synuclein peptide vaccine. Mov Disord 37(7):1416–1424
Zhang H, Li Y, Yu J, Guo M, Meng J, Liu C, Xie Y, Feng L, Xiao B, Ma C (2013) Rho kinase inhibitor fasudil regulates microglia polarization and function. NeuroImmunoModulation 20(6):313–322
Zhang L, Zhang L, Li L, Hölscher C (2019) Semaglutide is neuroprotective and reduces α-synuclein levels in the chronic MPTP mouse model of Parkinson’s disease. J Parkinsons Dis 9(1):157–171
Zhao Y, Zhang Q, Xi J, Li Y, Ma C-G, Xiao B-G (2015) Multitarget intervention of Fasudil in the neuroprotection of dopaminergic neurons in MPTP-mouse model of Parkinson’s disease. J Neurol Sci 353(1–2):28–37
Zimmermann M, Brockmann K (2022) Blood and cerebrospinal fluid biomarkers of inflammation in Parkinson’s disease. J Parkinsons Dis 12(s1):S183–S200
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PL has received consulting fees from AbbVie, Alexion, BIAL, Desitin, ITF Pharma, STADA Pharm, Woolsey Pharmaceuticals and Zambon. The other authors declare no competing interests with regard to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolff, A., Schumacher, N.U., Pürner, D. et al. Parkinson’s disease therapy: what lies ahead?. J Neural Transm 130, 793–820 (2023). https://doi.org/10.1007/s00702-023-02641-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02641-6