Abstract
The dementia spectrum encompasses a range of disorders with complex diagnosis, pathophysiology and limited treatment options. Positron emission tomography (PET) imaging provides insights into specific neurodegenerative processes underlying dementia disorders in vivo. Here we focus on some of the most common dementias: Alzheimer’s disease, Parkinsonism dementias including Parkinson’s disease with dementia, dementia with Lewy bodies, progressive supranuclear palsy and corticobasal syndrome, and frontotemporal lobe degeneration. PET tracers have been developed to target specific proteinopathies (amyloid, tau and α-synuclein), glucose metabolism, cholinergic system and neuroinflammation. Studies have shown distinct imaging abnormalities can be detected early, in some cases prior to symptom onset, allowing disease progression to be monitored and providing the potential to predict symptom onset. Furthermore, advances in PET imaging have identified potential therapeutic targets and novel methods to accurately discriminate between different types of dementias in vivo. There are promising imaging markers with a clinical application on the horizon, however, further studies are required before they can be implantation into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
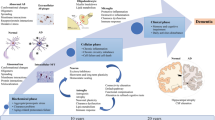
Neurodegenerative dementia spectrum disorders are associated with progressive and irreversible neuronal loss. Dementia encompasses a wide range of disorders which are often difficult to accurately diagnose and there are limited treatments options. Positron emission tomography (PET) is a molecular imaging technique which has the potential to aid understanding of disease pathophysiology, differential diagnoses between dementia disorders, monitor disease progression and response to treatments (Politis et al. 2017; Rocchi et al. 2015; Politis and Piccini 2012; Politis 2014). The development of new PET tracers provides increasing ability to visualise distinct histopathologic hallmarks underlying different dementias (Table 1). Other neuroimaging techniques such as magnetic resonance imaging (MRI) have also been used to investigate dementia pathophysiology including regional brain atrophy, changes in white matter integrity and functional connectivity. This review provides an overview of advances in PET imaging across common dementias.
Dementia disorders can be classified according to their pathology of misfolded proteins including amyloid-β, tau and alpha-synuclein. Alzheimer’s disease (AD), the most common neurodegenerative dementia disorder, is pathologically characterised by extracellular amyloid plaques composed of amyloid-β, and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein (Hardy 2006). AD usually presents with difficulties remembering autobiographic events and to a lesser extent by problems with language, executive and visuospatial functions (McKhann et al. 2011). Mild cognitive impairment (MCI) can represent a transitional stage between healthy and AD with 12–15% progressing from MCI to AD each year (Petersen et al. 2009). MCI patients present with mild memory impairments on cognitive testing but are often not affecting their daily function.
Frontotemporal lobe degeneration (FTLD) is associated with aggregated tau, as Pick bodies, and encompasses several distinct pathologies involving frontal and/or anterior temporal lobe degeneration alongside associated dementia (Rabinovici and Miller 2010). Frontotemporal lobar degenerative disorders include behavioural frontotemporal dementia (FTD), semantic dementia and progressive non-fluent aphasia (Olney et al. 2017).
Degenerative parkinsonian disorders can be broadly divided into two groups based on neuropathological characteristics: (1) synucleinopathies, which includes PD with dementia (PDD), dementia with Lewy bodies (DLB) and multiple system atrophies (MSAs); and (2) tauopathies, including progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS), associated with intra-neuronal and astrocytic aggregates of tau. The neuropathology of synucleinopathies is characterised by the presence of alpha-synuclein, which form the main component of Lewy bodies and neurites (Spillantini et al. 1997). Histopathological evidence suggests that alpha-synuclein often coexists with amyloid-β plaques and tau neurofibrillary tangles (Compta et al. 2014; Horvath et al. 2013). Such neuropathological changes could result in disruption of the dopaminergic, cholinergic and noradrenergic neuromodulating projecting networks as well as local or regional neuronal and synaptic dysfunction. In vivo molecular imaging tools could help to elucidate the complex interaction between PD-like (alpha-synuclein) and AD-like (amyloid-β plaques and tau) pathologies and their role in the development of cognitive impairment.
Generally cognitive deficits in parkinsonian dementia syndromes relate aberrant information processing compared to AD which typically involves more content-specific deficits in language and perceptual processing. Dementia is common in PD patients with over 80% of patients developing dementia after 20 years of the disease (Aarsland et al. 2008, 2017). PDD and DLB are clinically distinguished based only on whether Parkinsonism or dementia developed first (McKeith et al. 2005; Niccolini and Politis 2016). Patients with levodopa-responsive parkinsonism who develop dementia more than 1 year after the onset of cardinal parkinsonism motor symptoms are classified as PDD; while patients who develop dementia and parkinsonism within 1 year or when the onset of dementia precedes parkinsonism are classified as DLB.
Glucose metabolism: FDG PET imaging
[18F]fluorodeoxyglucose (FDG) PET studies are used to estimate the local cerebral metabolic rate of glucose consumption; providing information on the distribution of neuronal death and synaptic dysfunction with relatively disease-specific reduction patterns (Magistretti 2000).
FDG-PET in Alzheimer’s disease
FDG–PET plays a key role in early and differential diagnosis of AD due to unique patterns of hypometabolism (reduced FDG uptake). Retrospective studies have illustrated FDG–PET has 94% sensitivity and 73% specificity to predict AD pathology (Silverman et al. 2001). In early stages of AD, FDG–PET has revealed hypometabolism in the parietotemporal association cortices, posterior cingulate and precuneus regions (Minoshima et al. 1995). Hypometabolic regions spread to the frontal association cortices in moderate-to-severe AD, while metabolism in the striatum, thalamus, primary sensorimotor cortices, visual cortices and cerebellum are relatively persevered through disease progression. Compared to late-onset AD, patients with early-onset experience greater hypometabolism in parietal and posterior cingulate cortices and precuneus regions to reach the same severity of dementia (Kim et al. 2005). Lower FDG uptake is associated with lower cognitive function measures such as the Mini-Mental State Examination (MMSE) and specific word recognition tests (Landau et al. 2011). Moreover, glucose metabolism has been suggested as a sensitive marker for predicting future cognitive decline (Landau et al. 2011; Zhang et al. 2012; Arbizu et al. 2013). Recently, it has been reported that hypertension in AD patients is associated with worse cognitive function and reduced glucose hypometabolism in the hippocampus (Moonga et al. 2017).
There is evidence to suggest changes in glucose metabolism occur early in preclinical AD stages. In autosomal dominant AD, patterns of hypometabolism presenting up to 10 years before symptom onset are consistent with patterns observed in established AD (Kennedy et al. 1995; Bateman et al. 2012). Similar patterns were also observed in cognitively normal apolipoprotein (APOE) e4 carriers who have an increased risk of developing AD (Langbaum et al. 2010; Knopman et al. 2014). FDG-PET studies in MCI have demonstrated similar patterns of glucose reduction as in early AD, with very early metabolic deficits in the medial portion of the parietal cortex, posterior cingulate and spreading to temporal and prefrontal cortices (Small et al. 2008). FDG–PET studies have shown patterns of hypometabolism in the inferior parietal lobe, precuneus and posterior cingulate can predict the conversion from MCI to AD (Yuan et al. 2009). Moreover, FDG–PET was found to have higher accuracy for predicting conversion of MCI to AD than structural MR imaging (Yuan et al. 2009).
Multimodal imaging studies, using FDG PET and amyloid PET have investigated the relationship between amyloid plaque deposition and glucose metabolism. These studies have produced conflicting results with some showing an association between local amyloid plaque load and hypometabolism (Lowe et al. 2014; Engler et al. 2006; Cohen et al. 2009; Edison et al. 2007) and others showing no correlation (Li et al. 2008; Furst et al. 2012; Rabinovici et al. 2010). Discrepancies in findings could be due to changing relationship between amyloid plaque load and glucose metabolism depending on disease stage or brain region. A recent study suggests cortical hypometabolism is mainly linked to increased global, rather than regional, amyloid burden (Altmann et al. 2015). Further longitudinal studies are required to clarify mechanisms underlying the relationship between glucose metabolism and amyloid plaque load.
FDG-PET in parkinsonian dementias
Patterns of hypometabolism in DLB are similar to those observed in AD, with early involvement of temporal lobes extending to parietal and frontal areas. However, in DLB reduced metabolism also occurs in the occipital primary and association areas (Minoshima et al. 2001; Ishii et al. 1998). Patients with PDD showed greater metabolic reduction in the visual cortex and persevered metabolism in medial temporal cortex compared to AD patients (Vander Borght et al. 1997). Therefore, occipital hypometabolism is a marker to distinguish between patients with PDD or DLB and AD with 90% sensitivity and 80% specificity (Minoshima et al. 2001). Decreased metabolic activity in the anterior cingulate is potentially greater in DLB than in PDD (Yong et al. 2007); however, clear differentiation between PDD and DLB has proven difficult due to mostly similar metabolic patterns. Therefore, while FDG–PET can aid differential diagnosis between parkinsonian dementia (PDD or DLB) and dementia due to AD, is not able to clearly differentiate between PDD and DLB.
Combined PET imaging of amyloid-β plaques and FDG showed parietotemporal and occipital metabolism was significantly lower in patients with DLB compared to AD (Ishii et al. 2015; Kantarci et al. 2012). Furthermore, there was no difference in regional glucose metabolism between amyloid-β positive and amyloid-β negative DLB patients. This suggests that regional hypometabolism in DLB is independent of AD amyloid-β pathology, with hypometabolism is much higher in DLB than AD (Ishii et al. 2015; Kantarci et al. 2012; Fujishiro et al. 2010). Therefore, the presence of hallmark alpha-synuclein pathology could contribute to the majority of cognitive decline and regional hypometabolism in DLB.
In early stages of PSP, decreased glucose metabolism in the midbrain, lateral and medial frontal lobes and caudate nucleus has been reported (Juh et al. 2005, 2004) in FDG-PET studies which can distinguish PSP from other parkinsonism diseases (Hosaka et al. 2002; Klein et al. 2005). CBS patients with symptoms of dementia show an AD-like pattern of hypometabolism but with asymmetric glucose metabolic reduction in the lateral frontal, lateral temporal and medial and lateral parietal cortices, the pre- and post-central gyri and thalamus (Hirono et al. 2000). A comparison between CBS and PSP revealed relative hypometabolism in the thalamus and mid-brain in PSP patients and the parietal lobe in CBS patients (Juh et al. 2005).
FDG-PET in frontotemporal lobar degenerative disorders
In FTD, frontal and temporal regions, as well as the striatum and thalamus show decreased glucose metabolism (Ishii et al. 1998a, b). Metabolic and morphological changes occur in bilateral frontal and temporal lobes, whereas regions of metabolism are more severely affects than regions of atrophy in the frontal lobe (Engler et al. 2008). As the disease progresses to advanced stages, hypometabolism spreads from localised frontal lobe areas to the parietal and temporal cortices (Diehl-Schmid et al. 2007). FDG-PET can be used to aid clinical diagnosis of FTLD, using a visual rating scale of cerebral metabolism, with a sensitivity of 89% (Poljansky et al. 2011). FTD and semantic dementia present with distinct patterns of cerebral metabolism. FTD is associated with frontal hypometabolism, whereas patients with semantic dementia have severe hypometabolism in the temporal lobe (Diehl et al. 2004). Furthermore, patients with FTLD have significantly lower metabolism in the frontal lobe and basal ganglia compared to AD and MCI (Poljansky et al. 2011). Therefore, FDG-PET could be used to differentiate between diagnosis of FTLD, AD and MCI.
Amyloid PET imaging
[11C]Pittsburgh compound-B (PIB) has been the most widely used PET tracer for amyloid imaging over the past decade. However, the short half-life of [11C]PIB, requiring an onsite cyclotron and synthesis system, limits its clinical use. Therefore, second generation 18F-labelled amyloid tracers, with longer half-life, such as 18F-AV-45 (florbetapir) (Okamura and Yanai 2010) and [18F]AZD4694 (Cselenyi et al. 2012), [18F]florbetaben (Fodero-Tavoletti et al. 2012) and [18F]flutemetamol (Vandenberghe et al. 2010), have greater potential to aid accurate diagnosis and evaluation of disease-modifying therapies in dementia disorders. PIB and 18F-labelled PET tracers bind with high affinity to the β sheet structure of fibrillary amyloid; thus, binding specifically to amyloid-β peptide aggregates and not other misfolded proteins, such as tau or α-synuclein (Fodero-Tavoletti et al. 2012).
Amyloid imaging in Alzheimer’s disease
PIB–PET studies have demonstrated PIB retention consistent with post-mortem distribution of amyloid-β plaques (Ikonomovic et al. 2008). In clinical practice, visual analysis of amyloid PET provides binary information on the presence or absence of amyloid load in individuals with high sensitivity and specificity (Teipel et al. 2015). Longitudinal studies have shown that PIB–PET is able to detect the presence of amyloid-β plaques many years before the clinical disease onset in autosomal dominant AD (Benzinger et al. 2013). PIB has also been shown to predict cognitive decline in healthy individuals and patients with amnestic mild cognitive impairment (aMCI) (Okello et al. 2009; Lim et al. 2014). Furthermore, [18F]florbetapir has been shown to be predictive of cognitive deterioration over a 3 year follow up in patients with MCI and probably AD (Doraiswamy et al. 2014). Disease-specific PET tracers currently available for clinical use to distinguish AD and MCI include tracers for amyloid-β plaques to show or exclude brain amyloid load; [18F]florbetapir, [18F]florbetaben and [18F]flutemetamol.
According to the amyloid cascade hypothesis, the accumulation of amyloid-β is an early preclinical pathological process preceding cognitive decline (Jack et al. 2010). While amyloid imaging may play a role in diagnosis of AD, amyloid PET imaging does not correlate with cognitive decline and is weak in monitoring progression once early AD is established (Engler et al. 2006). Moreover, amyloid-β immunotherapies do not suppress dementia progression unless the drug also diminishes tau pathology (Karran and Hardy 2014). Thus, amyloid deposition may have already peaked in a patient with MCI and reached a plateau by early AD. Amyloid deposition is also not unique to AD and can be found in healthy elderly people. [11C]PIB PET has shown approximately one-third of healthy elderly individuals show increased amyloid deposition (Pike et al. 2007; Aizenstein et al. 2008). Therefore, clinical impairment may be due to, in part, the ability of individuals to tolerate aggregated amyloid. Genetic risk factors, lifestyle choices, environmental factors and neuropathological comorbidities could alter the threshold for the onset of cognitive impairment associated with amyloid-β aggregation (Bennett et al. 2006; Schneider et al. 2009). Studies have shown that APOE genotype differentially effects the distribution of amyloid plaques in AD (Ossenkoppele et al. 2013). The prevalence of amyloid pathology in people without dementia is associated with age, APOE genotype and presence of cognitive impairment. Moreover, it is suggested that there is a 20- to 30-year interval between first development of amyloid positivity and onset of dementia (Ossenkoppele et al. 2015; Jansen et al. 2015).
Going forward, the role of amyloid PET in clinical practice is in early stages of dementia to predict, on an individual basis, the risk of disease conversion from MCI to AD. However, amyloid PET alone is unlikely sufficient to accurately represent timings MCI to AD conversion.
Amyloid imaging in parkinsonian dementia
Amyloid deposits, assessed with PIB-PET, have been associated with AD-like atrophy in patients with DLB and PDD (Shimada et al. 2013). Amyloid deposition occurs early in DLB and may account in for the early presentation of dementia. Amyloid burden is higher in patients with DLB compared to PDD, but not as high as PIB levels observed in AD (Fujishiro et al. 2010; Gomperts et al. 2008). PDD may be differentiated from DLB by its relatively lower amyloid plaque load (Brooks 2009). PIB binding has been linked to cognitive impairment in DLB (Gomperts et al. 2012). In PDD typically PIB binding does not differ from PD patients or healthy individuals (Gomperts et al. 2008). However, cortical PIB retention has been shown to predict cognitive decline in PD (Gomperts et al. 2016). Therefore, amyloid deposition may contribute to the timing of dementia onset in Lewy body disorders.
Amyloid imaging in frontotemporal lobar degenerative disorders
PIB PET studies have revealed FTD patient’s exhibit no PIB retention; therefore, supporting PIB PET as a tool to differentiate between FTD and AD (Engler et al. 2008; Rabinovici et al. 2007). The identification of PIB-positive patients with frontotemporal lobe degeneration could suggest a mimicking of AD pathology. Furthermore, amyloid PET imaging can also be used to differentiate the diagnosis of semantic dementia from AD (Drzezga et al. 2008).
Tau imaging
Advances in the development of PET tracers selective for tau have, for the first time, enabled in vivo evaluation of tau pathology in dementias. First generation tau selective PET tracers include [18F]AV1451 (also known as [18F]T807) (Xia et al. 2013; Marquie et al. 2015) and THK series: [18F]THK523 (Fodero-Tavoletti et al. 2011), [18F]THK5105 (Okamura et al. 2014), [18F]THK5117 (Harada et al. 2015; Jonasson et al. 2016), [18F]THK5351 (Harada et al. 2016). These tau tracers bind to NFTs with higher affinity than amyloid-β fibrils (Fodero-Tavoletti et al. 2011). However, first generation tau tracers have limitations due to off-target binding to monoamine oxidase (MAO)-A/B. Furthermore, [18F]AV1451, [18F]THK5117 and [18F]THK5105 have substantial retention in striatal regions even in cognitively normal subjects which is inconsistent with neuropathological reports and could lead to overestimation of tau burden in adjacent cortical areas (Serrano-Pozo et al. 2011). First generation tau PET tracers predominantly detect AD like tau pathology, paired helical filaments with a mixture of 3-repeat (3R) and 4-repeat (4R) tau isoforms. The ability of [18F]AV1451 to detect 4R-tau isoforms, predominant in CBS and PSP, has been debated with autoradiography studies showing lower binding of [18F]AV1451 in non-AD brains compared to AD (Marquie et al. 2015; Sander et al. 2016; Lowe et al. 2016).
Advances in novel second-generation tau radioligands, [18F]PI-2620 and [18F]MK-6240, with no off-target binding have greater potential to aid accurate diagnosis and evaluation of disease-modifying therapies. [18F]MK-6240 has higher affinity for neurofibrillary tangles in AD brains than [18F]AV1451, greater uptake contrast between the hippocampus and cortical regions and no off-target binding to MAO-A/B (Hostetler et al. 2016; Walji et al. 2016; Collier et al. 2017; Lohith et al. 2016). Preliminary results from [18F]PI-2620 are promising with low affinity for MAO-A/B and strong binding to both tau isoforms, 3R-tau in Pick’s disease and 4R-tau in PSP (Mueller et al. 2017; Barret et al. 2017). Thus, with specificity for 3R and 4R tau, [18F]PI-2620 has the potential to quantify tau pathology in vivo in AD patients as well as other dementias with prominent 4R-tau pathology such as PSP and CBS. Advances in new tau PET tracers provide opportunities to investigate regional distribution of tau pathology, clinical relevance to dementia and parkinsonian disorders, support early differential diagnosis and monitoring disease progression.
Tau imaging in Alzheimer’s disease
Unlike amyloid-β deposition which accumulate early in AD and plateaus by symptom onset, intracellular neurofibrillary tangles (NFT) formation starts later and spreads throughout the course of the disease (Serrano-Pozo et al. 2011). Moreover, neuropathological studies show accumulation of NFT correlates with neuronal dysfunction and symptom progression (Serrano-Pozo et al. 2011; Arriagada et al. 1992; Bierer et al. 1995a, b). Distinct timing and distribution of amyloid-β plaques and tau suggests they aggregate independent, however, their pathological relationship is complex (Ittner and Gotz 2011). It is hypothesised that tau pathology is more closely linked to symptomatology and patterns of glucose hypometabolism in AD.
Early PET studies with [18F]THK523 showed higher retention in temporal, parietal and orbitofrontal cortices and in the hippocampus in AD patients compared to healthy controls (Villemagne et al. 2014). However, high white matter retention of [18F]THK523 hinders accurate visual interpretation of signals, thus preventing use in research or clinical settings (Villemagne et al. 2014). [18F]THK5105 and [18F]THK5117 showed higher binding affinity than [18F]THK523 (Okamura et al. 2005). PET studies with improved derivatives, [18F]THK5105 and [18F]THK5117, revealed binding in the temporal lobe clearly differentiating AD patients from healthy elderly subjects, and distinct from amyloid-β PET imaging with PIB (Okamura et al. 2014; Harada et al. 2015). Furthermore, [18F]AV1451 PET demonstrated cortical retention consistent with the known distribution of tau (Xia et al. 2013; Arnold et al. 1991; Schwarz et al. 2016) and a strong association with dementia severity and cognitive impairment (Ossenkoppele et al. 2016; Cho et al. 2016) (Fig. 1). In vivo studies show [18F]AV1451 uptake in AD patients is compatible with Braak staging (Schwarz et al. 2016). Therefore, supporting evidence from post-mortem and animal studies suggesting that aggregation of tau is closely linked to patterns of neurodegeneration and clinical manifestations of AD.
PET with [18F]AV1451 and [18F]AV45 showing differentiate uptake patterns of tau and amyloid pathology respectively in Mild Cognitive Impairment (MCI), Alzheimer’s Disease (AD) and Corticobasal Syndrome (CBS). MCI and AD subjects show increased [18F]AV1451 in temporal cortices, while CBS subject shows asymmetrical uptake in frontal and parietal regions. MCI and AD show positive [18F]AV45 scans with widespread amyloid binding while CBS subject has no amyloid pathology. MCI patient 77 years of age, MMSE = 28, MoCA = 15; AD patient 75 years of age, MMSE = 25, MoCA = 15; and CBS patients 75 years of age, MMSE = 17, MoCA = 15. AD Alzheimer’s Disease, CBS Corticobasal Syndrome, MCI Mild Cognitive Impairment, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment
The use of PET as a tool to quantify tau pathology has been investigated to monitor conversion from MCI to AD. [18F]THK5117, [18F]THK5351 and [18F]AV1451 have shown highest uptake in AD followed by MCI, compared with low signal in normal controls (Harada et al. 2015), with uptake correlating with cognitive impairment (Chien et al. 2013). Moreover, a study showed tau signal increasing over 18 months in MCI and AD as symptoms progress (Mintun et al. 2015). In MCI and healthy subject’s association cortex tau binding was higher in amyloid-positive than amyloid-negative individuals (Pontecorvo et al. 2015). Regional tau correlated with mean cortical PIB binding in healthy controls, AD and MCI (Johnson et al. 2016). A [11C]PBB3 PET study also showed signal retention in the hippocampus in AD which spreads to association cortex as disease progresses (Maruyama et al. 2013). PET with [18F]AV1451 showed increased binding in MCI compared to controls in association cortical areas (Johnson et al. 2014). Therefore, tau PET imaging could provide a valid tool to monitor disease progression and cognitive decline in AD.
To understand the relationship between tau and amyloid-β pathology, tau deposition in healthy cognitively normal subjects has also been investigated with tau PET imaging. Cognitively normal subjects showed increasing accumulation of tau in basal ganglia, hippocampus, midbrain and fornix with ageing (Schultz 2015). Moreover, amyloid-β load and tau binding was observed in the inferior temporal lobe as well as an association between tau binding and rate of amyloid-β accumulation. These findings are consistent with histological findings that amyloid-β pathology can influences tau spreading throughout the cortex (Schultz 2015). It has been suggested that hippocampal tau is an age-related process, independent of AD but amplified by amyloid precursor protein dysfunction (Delacourte et al. 2002; Crary et al. 2014). Further studies combining tau and amyloid-β PET imaging could help to confirm whether the accumulation of amyloid-β triggers and accelerates the spread of tau deposition outside the mesial temporal cortex.
Preliminary results from [18F]PI-2620 demonstrate high signal in brain regions of known tau pathology in AD and distinct uptake patterns in PSP compared to AD (Barret et al. 2017). A pilot study with [18F]MK-6240 in four healthy controls and five AD patients, showed high uptake of [18F]MK-6240 in regions associated with NFT deposition in AD and negligible tracer binding in healthy individuals (Lohith et al. 2017).
Tau imaging in parkinsonian dementia
Tau PET imaging in has the potential to differentiate DLB from PD and other parkinsonian tauopathies, due to patterns of tau disruption, and shed light on how tau aggregates contribute to cognitive decline in these disorders. To date, PET imaging has been employed to investigate tau deposition in DLB, PSP and CBS. Preliminary studies in PSP and CBS show [11C]PBB3 uptake in neocortical and subcortical structures in CBS patients (Shimada et al. 2015). This is suggestive that PBB3 might bind to non-AD tau conformations. However, a radiolabelled metabolite of PBB3 also crosses the blood–brain barrier making quantification less reliable. Therefore, the development of more selective derivatives is required.
While in vitro studies have shown [18F]AV1451 binds predominately to paired helical filament (PHF) of 3R-tau (Marquie et al. 2015) in AD brains, autoradiography studies in post-mortem CBS brains show low, but specific, [18F]AV1451 binding (Marquie et al. 2015; Sander et al. 2016; Lowe et al. 2016). Recently, [18F]AV1451 PET studies have shown tracer retention patterns in CBS patients matching the known distribution of tau pathology with high uptake in frontal and parietal cortical regions (Cho et al. 2017; Smith et al. 2017) (Fig. 1). Furthermore, increased uptake of [18F]AV1451, in the absence of amyloid, was shown to correlate with post-mortem levels of 4R-tau burden in two autopsy-confirmed CBS patient (Josephs et al. 2016; McMillan et al. 2016). In CBS patients [18F]AV1451 uptake was higher contralateral to the side with greater cortical dysfunction and parkinsonism (Cho et al. 2017; Smith et al. 2017). PET with [18F]THK5351 has also been studies to detect tau pathology in CBS. [18F]THK5351 uptake was higher in frontal, parietal and the globus pallidus in five CBS patients compared to healthy controls, with higher binding contralateral to the clinically most affected side (Kikuchi and Neurology 2016). Therefore, indicating the usefulness of [18F]AV1451 and [18F]THK5351 PET as markers to detect tau pathology in CBS.
Since [18F]AV1451 does not bind to Lewy bodies or TDP43 aggregates (Gomperts et al. 2015), it has been used to investigate tau levels in PSP and CBS. In PSP, increased [18F]AV1451 uptake has been reported in the striatum, pallidum, thalamus and frontal cortex (Whitwell et al. 2017; Passamonti et al. 2017). A study comparing uptake patterns of [18F]AV1451 in PSP and PD report distinct subcortical binding patterns, with greater subcortical binding in PSP and lower binding in the substantia nigra in PD compared to controls; however, there was no correlation between [18F]AV1451 binding and severity of motor dysfunction and no increased binding in cortical regions in PSP. Therefore, indicating [18F]AV1451 PET might not be optimal for assessing tau pathology in vivo in PSP. Preliminary results from the second generation tau tracer [18F]PI-2620 showed different and specific uptake patterns in AD and PSP, with no off-target binding, indicating its potential to aid differential diagnosis (Mueller et al. 2017; Barret et al. 2017).
Recently, two studies shave investigated patterns of tau distribution have been in patients with DLB (Gomperts 2016; Kantarci et al. 2017). Increased [18F]AV1451 uptake has been reported in the posterior temporoparietal, occipital cortex and precuneus in DLB compared to healthy controls (Gomperts 2016; Kantarci et al. 2017). Increased [18F]AV1451 uptake in the medial temporal cortex was able to distinguish AD dementia from probable DLB (Kantarci et al. 2017). Furthermore, a cross-sectional studies have compared [18F]AV1451 and [11C]PIB uptake in patients with DLB compared to PD with cognitive impairments (CI) and cognitively normal PD patients (Gomperts 2016), and patients with AD (Kantarci et al. 2017). In patients with DLB and PD with cognitive impairment, increased cortical [18F]AV1451 uptake, particularly in inferior temporal and precuneus regions, was observed compared to uptake in healthy controls (Gomperts 2016). Whereas PD patients without cognitive impairment did not show increased tau depositions compared to healthy controls. Increased tau deposition was observed in DLB patients with low cortical amyloid burden (Gomperts 2016). The presence of tau deposits with minimal amyloid load, indicated tau pathology is possible without amyloid in DLB. Furthermore, increased [18F]AV1451 uptake, but not [11C]PIB retention, in the inferior temporal gyrus and precuneus correlated with greater cognitive impairment in DLB and PD patients with cognitive impairment (Gomperts 2016).
Tau imaging in frontotemporal lobar degenerative disorders
In FTD, PET with [18F]AV1451 has shown tracer binding in frontal, insular and anterior temporal cortex (Raboinovici et al. 2015). A single-case study of a patient with behavioural variant FTD showed increased [18F]AV1451 in the anterior temporal lobes and anterior cingulate cortex (Bevan Jones et al. 2016), regions known for tau accumulation in FTD (Kertesz et al. 2005). Furthermore, recently [18F]AV1451 PET has been shown to characterise tau pathology in a patient with FTD associated with the microtubule-associated protein tau (MAPT) mutation. [18F]AV1451 uptake was greatest in frontal, temporal and cingulate regions, and cortical binding correlated with cortical brain atrophy (Spina et al. 2017). Increased [18F]FDDNP uptake has also been reported in frontal and lateral temporal regions in FTLD compared to controls, similar to uptake observed in AD; however, in FTLD uptake in the parietal cortex is lower than in AD (Small et al. 2006). Therefore, [18F]FDDNP and [18F]AV1451 PET could provide a tool for evaluating the presence of tau deposits in FTD and to aid the differential diagnosis of FTLD from AD.
Alpha-synuclein imaging
Alpha-synuclein is the defining component of Lewy bodies which are characteristic of PDD, DLB and FTD. Alpha-synuclein is an important target to understand the pathophysiology of neurodegenerative dementias and is a potential treatment target. The presence of α-synuclein has been implicated in mitochondrial and proteasomal dysfunction and vesicle trafficking within presynaptic dopaminergic neurons (Burre et al. 2010).
Alpha-synuclein is an amyloidogenic protein; therefore, PET tracers such as [11C]PIB and [18F]BF227 originally developed to target amyloid-β plaques have been investigated for their potential affinity to α-synuclein. There is some evidence of PIB binding to aggregated α-synuclein, its binding affinity was much higher for amyloid-β plaques. Moreover, preclinical PET data yielded inconsistent findings (Maetzler et al. 2008; Ye et al. 2008). Immunohistochemical studies demonstrated [18F]BF227 binding to Lewy bodies in PD brain sections (Fodero-Tavoletti et al. 2009). Recently, a novel α-synuclein tracer [123I]SIL23 (Bagchi et al. 2013) has been developed which binds to α-synuclein fibrils in post-mortem PD brain tissue. The distribution of SIL23 correlated with known levels of fibrillary α-synuclein in PD. Unfortunately, high non-specific binding in white matter limits the use of [123I]SIL23 in imaging studies.
To-date all tested compounds bind to other molecules, most commonly amyloid-β, and are, therefore, not suitable as selective tracers for the specific in vivo quantification of α-synuclein pathology. Work is on-going to generate a viable radiotracer for detecting α-synuclein in vivo which could serve to aid earlier and accurate diagnosis, and enable disease monitoring of future therapeutics.
Cholinergic PET imaging
The cholinergic system is hypothesised to play a key role in cognitive processing and has a pivotal role in pathophysiology of dementias (Berger-Sweeney 2003). PET imaging has been employed to understand pre- and post-synaptic cholinergic dysfunction in dementia spectrum disorders, using markers for presynaptic choline acetyltransferase (ChAT) and acetylcholinesterase (AChE), and post-synaptic muscarinic acetylcholine receptors (mAChR) and nicotinic acetylcholine receptors (nAChRs) (Roy et al. 2016).
Cholinergic imaging in Alzheimer’s disease
The cholinergic hypothesis states that degeneration of cholinergic neurons and subsequent decreased cholinergic neurotransmission leads to several cognitive and functional impairments in AD (Bartus et al. 1982). PET studies with [11C]PMP, a selective tracer for AChE (Irie et al. 1994), have shown reduced AChE activity (Kuhl et al. 1999) in the cortex, hippocampus and amygdala consistent with widespread ChAT and AChE loss observed in AD post-mortem studies (Perry et al. 1977). Decreased AChE was associated with cognitive impairments and in particular with functions of attention and working memory, instead of episodic memory, in AD (Bohnen et al. 2005). In APOE ε4 carriers, [11C]MP4A PET showed lower reductions in cortical AChE activity compared to non ε4 carriers (Eggers et al. 2006) suggesting APOE4 allele provides a protective role against widespread AChE activity loss. However, potential mechanisms underlying this are unclear. Recent development of the PET tracer [18F]FEOBV provides a promising tool to further assess the role of presynaptic vesicular acetylcholine transport (VAChT) dysregulation in AD (Petrou et al. 2014).
PET tracers [18F]2FA, targeting α4β2 nAChR and [11C]nicotine have been employed to investigate post-synaptic cholinergic dysfunction in AD (Sabri et al. 2008). PET with [18F]2FA showed significant reductions in nAChR availability in frontal, parietal and temporal cortices and the hippocampus in AD which correlated with cognitive decline (Sabri et al. 2008). Moreover, loss of nAChR was also observed in amnestic MCI in medial temporal cortex (Terriere et al. 2010), indicating PET with [18F]2FA may provide a tool for predicting conversion from MCI to AD. However, other studies have reported preservation of nAChRs in AD or MCI compared to healthy controls (Mitsis et al. 2009). Therefore, further studies are required to fully understand how early in the disease process cholinergic dysfunction occurs and the potential use for predicting conversion MCI to AD. Development of PET radioligands, such as [18F]AZAN, with faster regional kinetics than [18F]2FA, could aid further understanding of cholinergic dysfunction in AD (Horti et al. 2010). PET with [11C]nicotine has shown loss of nAChR binding in the frontal and temporal cortices and the hippocampus in moderate AD (Nordberg et al. 1995). As with presynaptic cholinergic markers, [11C]nicotine binding also correlated with attention deficit but not episodic memory or visuospatial impairment (Kadir et al. 2006). However, [11C]nicotine tracer has high levels of nonspecific binding and depends strongly on cerebral blood flow (Nyback et al. 1994).
Negative correlations have been reported between ChAT and nAChR availability and amyloid-β plaque load (Perry et al. 1977; Bierer et al. 1995; Okada et al. 2013); while reduction in AChE did not correlate with hypometabolism in posterior cingulate and parietal regions as measured with FGD-PET (Kuhl et al. 1999, 1996). Thus, suggesting increasing amyloid-β burden may induce degeneration of cholinergic neurons while different pathophysiological mechanisms could underlie cholinergic and metabolic decline in AD.
Acetylcholinesterase inhibitors are the main symptomatic treatment to improve cholinergic neurotransmission and cognitive function in AD (Declercq et al. 2016). PET studies before and after treatment with donepezil or rivastigmine, showed up to 40% frontal cortical inhibition of AChE activity. Clinical trials report modest improvement in behavioural and attentional symptoms in AD patients treated with AChE inhibitors (Kaasinen et al. 2002; Shinotoh et al. 2004). Following galantamine treatment reduced cortical [11C]PMP signal was observed indicating galantamine treatment increased ACh concentrations subsequently leading to increased cholinergic neurotransmission (Kadir et al. 2008). Clinical trials investigating the potential role of mAChR agonists/antagonists to ameliorate cognitive symptoms in AD are on-going (Declercq et al. 2016).
Cholinergic imaging in parkinsonian dementias
In addition to loss of dopaminergic function other neurotransmitter systems, including cholinergic dysfunction, are involved in the pathophysiology of PD. Cholinergic PET imaging studies with [11C]MP4A or [11C]PMP uptake, has shown that loss of cortical AChE activity occurs early in PD and is more apparent in PDD than in non-demented PD (Bohnen et al. 2003; Hilker et al. 2005; Shimada et al. 2009). Lower cortical AChE activity was associated with reduced cognitive performance scores for attention, memory and executive functions (Bohnen et al. 2003) but not with severity of motor symptoms (Bohnen et al. 2006). This is consistent with post-mortem evidence suggesting loss of forebrain cholinergic function is associated with PDD (Hilker et al. 2005; Shimada et al. 2009; Bohnen et al. 2003). Loss AChE activity occurs early in PD becoming more widespread in PDD and DLB (Shimada et al. 2009). PDD patients exhibit higher cholinergic dysfunction than AD patients, matched according to degree of cognitive impairment, suggesting different mechanisms may underlie cognitive decline in PD and AD (Bohnen et al. 2003). Moreover, unlike in AD, thalamic AChE activity was decreased in PDD patients (Kotagal et al. 2012).
Differing cortical AChE activity in PSP and PD indicates potential use of cholinergic imaging markers in differential diagnosis (Gilman et al. 2010). Unlike in PD, PSP patients exhibit reduced thalamic [11C]MP4A uptake while striatal cholinergic neurons are unaffected (Mazere et al. 2012). CBS patients exhibit reduced AChE in paracentral, frontal, parietal and occipital cortices (Hirano et al. 2010). Combined, findings support the role of cholinergic dysfunction in PSP, CBS and PDD and potential therapeutic use of cholinesterase inhibitor (Matsunaga 2015).
Cholinergic imaging in frontotemporal lobar degenerative disorders
PET studies showed no different in AChE activity in FTD patients compared to healthy controls (Hirano et al. 2010). This may explain why treatment with AChE inhibitors has proven ineffective (Mendez et al. 2007).
Imaging neuroinflammation
Neuroinflammation and microglia activation plays a central role in the neurodegeneration process. Microglial activation is observed in numerous neurodegenerative dementias with differing protein pathologies, including amyloid, tau and alpha-synuclein, suggesting that microglia activation is not specifically related to one particular protein pathology, rather reflects a common neurodegenerative process. Understanding microglia activation in vivo will likely provide insights into pathophysiology of dementias, as well as a potential marker of disease progression.
Activated microglia cells overexpress mitochondrial translocator protein (TSPO) which can be targeted by PET radioligands (Leung 2004; Politis et al. 2012). The first generation TSPO tracer, [11C]PK11195 is most commonly used to image microglia activation and neuroinflammation in vivo. However, [11C]PK11195 has several limitations, such as high levels of non-specific binding, low brain penetration and poor signal-to-noise ratio (SNR) (Petit-Taboue et al. 1991; Boutin et al. 2007). Therefore, second generation TSPO radioligands were developed, including [11C]PBR28, [11C]DAA1106, [11C]DPA-713, [18F]DPA-714, [18F]FEDAA1106, [18F]FEPPA, [18F]PBR06 and [18F]PBR111, which have improve specificity, affinity and SNR (Chauveau et al. 2009). However, second generation radioligands have been found to be sensitive to a single nucleotide polymorphism in the TSPO gene (rs6971) which affects tracer binding affinity resulting in heterogeneity in quantitative PET data. Recently, novel third generation TSPO radioligands [11C]ER176 (Ikawa et al. 2017) and [18F]GE180 (Fan et al. 2016) have been developed as insensitive to rs6971 polymorphism. Preliminary results indicate low brain uptake of [18F]GE180 compared with [11C]ER176 (Fan et al. 2016). Furthermore, [18F]GE180 is yet to be investigated in low-affinity binders (LABs). In humans, [11C]ER176 has high sensitivity for all rs6971 genotypes [high- (HABs), mixed- (MABs) and LABs], therefore, allowing quantification of TSPO levels in LAB subjects (Ikawa et al. 2017). Therefore, [11C]ER176 could be a potential radioligand to quantify microglial levels in dementia disorders in vivo.
Neuroinflammation in Alzheimer’s disease
Chronic neuroinflammation appears to play a central role in AD pathophysiology, and therefore, as a potential therapeutic target (Birch et al. 2014; Morales et al. 2014). PET studies with [11C]PK11195 show activated microglia in frontal, temporal, parietal, occipital and cingulate cortices in AD, with similar distribution pattern to that of amyloid- plaques (Edison et al. 2008). Moreover, increased cortical [11C]PK11195 binding was detected in about 60% of mild-to-moderate AD patients and 40% of MCI subjects (Cagnin et al. 2006). Detection of microglial activation in MCI suggests potential role for anti-inflammatory therapies in early disease stages (Okello et al. 2009). Association between the increased cortical [11C]PK11195 binding and MMSE suggests microglia activation is involved in neuronal dysfunction and cognitive impairment (Edison et al. 2008). However, not all studies detected increased [11C]PK11195 in MCI and mild-to-moderate AD but instead reported microglial activation limited to later stages of severe AD (Wiley et al. 2009). Discrepancies between results could be due to insensitivity of [11C]PK11195 to detect changes in microglia activation in mild-to-moderate AD, especially in studies with small sample sizes.
Second generation TSPO PET tracers, [11C]DAA1106 (Maeda et al. 2004), [18F]FEDAA1106 (Wang et al. 2012) have been used to assess microglia activation in AD (Yasuno et al. 2008). However, the affinity of these new tracers is dependent on which genetic polymorphism of TSPO is expressed (Kreisl et al. 2013). For example, binding to TSPO correlated with worse disease severity in AD (Kreisl et al. 2013). In AD patients without the rs6971 polymorphism, increased cortical [11C]PBR28 binding to TSPO has been reported but this cortical increase was not observed in MCI patients (Kreisl et al. 2013). Future studies with third generation PET tracers, such as [11C]ER176, which are insensitive to rs6971 polymorphism could provide further insights to the role of neuroinflammation in AD pathophysiology and monitor effective of potential disease-modifying therapies.
Neuroinflammation was also associated with increased accumulation of amyloid, measured with PET [11C]PIB, in AD and MCI (Kreisl et al. 2013). PET with [11C]PK11195 and [18F]FDG demonstrated significant inverse correlation between levels of microglial activation and cerebral glucose metabolic rate in the temporoparietal cortical regions (Fan et al. 2015). Thus, suggesting microglia activation has a detrimental effect on neuronal function. Microglia activation alongside amyloid deposition could cause neuronal damage and reduced glucose metabolism; while microglial activation and neuroinflammation could occur independently of amyloid deposition.
Neuroinflammation in Parkinsonian dementias
[11C]PK11195 PET in PDD has shown increased levels of microglia activation in the association cortex compared to non-demented PD patients, consistent with pathological studies showing involvement of the association cortex in PD and DLB (McGeer et al. 1993; Imamura et al. 2003; Iannaccone et al. 2013). In PDD, increased microglia activation has been reported in the cingulate, frontal, temporal and occipital cortical regions, as well as the striatum compared to healthy controls (Edison et al. 2013). Microglia activation also correlated with cognitive impairment; thus, microglial activation could be a factor driving disease progression in PDD. Association between microglia activation and hypometabolism in the superior frontal, superior and medial temporal, parietal and occipital lobes has been reported in PDD suggesting microglial activation is co-localised with hypometabolism (Fan et al. 2015).
Neuroinflammation in frontotemporal lobar degenerative disorders
Following recent discoveries of genes related to microglial activation, such as triggering receptor expressed on myeloid cells 2 (TREM2), in FTD (Rayaprolu et al. 2013) neuroinflammation is thought to play a role in FTD (Miller et al. 2013; Zhang 2015). Increased [11C]PK11195 binding in FTLD in the frontotemporal regions, suggests the presence of microglia reflecting progressive neuronal degeneration (Cagnin et al. 2004). A [11C]PK1195 PET study demonstrated microglial activation was higher in the temporal cortex in AD compared to FTLD, and greater in frontal subcortical white matter in FTLD compared to AD (Lant et al. 2014). This study showed higher temporal subcortical white matter in inherited FTLD with intronic mutations in MAPT compared to other genetic or non-genetic forms of FTLD (Lant et al. 2014). Thus, microglial involvement could serve as an in vivo marker of inherited FTLD–MAPT.
There are limited in vivo PET studies using second-generation TSPO PET tracers in FTD. A study using [11C]DAA1106 PET in pre-symptomatic MART gene carriers with parkinsonism linked to chromosome 17 (FTDP-17) showed increased microglial activation in frontal cortex compared to controls (Miyoshi et al. 2010). However, such increases were not overt throughout the brain in FTD. Further studies are required to track neuroinflammation in FTD and assessing the accuracy of TSPO imaging in detecting neuroinflammation in FTD. Early detection of neuroinflammation in FTD could identify novel treatment targets and tools for diagnosis and monitoring therapeutic efficacy.
Future directions and conclusion
Synaptic dysfunction and loss of synaptic proteins is thought to play an importance role in AD where synaptic loss is correlated with severity of dementia (Davies et al. 1987; Terry et al. 1991). However, the role of synapse loss in other types of dementia is less well established (Clare et al. 2010). Synaptic decline could be a common feature in pathological cascade associated with dementia as well as other neurodegenerative disorders. Therefore, the development of new PET tracers targeting specific synaptic proteins, such as [11C]UCB-J tracer for synaptic vesicle glycoprotein 2A (SV2A) (Finnema et al. 2016), could help to understand how early synaptic dysfunction occurs and its role in symptomatic development in different dementia disorders.
Astrocytes are a key component of the neuroinflammatory response. Activated following stimulation with proinflammatory mediators, astrocytes increase in proliferation, cell hypertrophy, and astroglial markers. One of these markers is the glial fibrillary acidic protein (GFAP), which is regulated by Imidazoline 2 binding sites (I2BS) (Maragakis and Rothstein 2001; Volterra and Meldolesi 2005). Specifically, I2BS are expressed selectively on astrocyte mitochondrial membranes and upregulation may play a fundamental role in the biology of reactive gliosis (Tyacke 2012; Garcia-Sevilla et al. 1998). Early pre-clinical data that have suggested the link between I2BS and neuroinflammation (Head and Mayorov 2006). Glial cells also protect against excitotoxicity by clearing excess glutamate from the extracellular space (Maragakis and Rothstein 2001). This protective function may have relevant to the selective neurodegeneration in dementia spectrum disorders. The I2BS are mainly located on astrocytes in the cortex, hippocampus, basal ganglia and brainstem (De Vos et al. 1994). Recently, the PET radioligand [11C]BU9908 has been developed for in vivo quantification of I2BS showing high specificity and selectivity (Tyacke 2012; Kealey et al. 2013; Parker et al. 2014). PET studies evaluating [11C]BU99008 expression could highlight role of astroglial activation in disease progression and associations with symptomatology.
Over the last decade, PET imaging has provided key insights into neurodegenerative dementia disorders, helped to identify therapeutic targets and monitor the effects of treatments. PET imaging has identified molecular changes at early and preclinical disease stages so as to monitor disease progression and predict timings of symptomatic conversion. Moreover, our understanding of how patterns of cognitive dysfunction, overlapping and distinct changes in dementia disorders has increased over the last decade. Continued advances in PET imaging techniques and the development of new tracers are crucial to understand pathophysiological changes and provide markers to accurately differential between dementia types. MRI and PET are likely to be most powerful when used in combination both for research and translation into clinical practice. In the next decade, advanced MRI techniques and PET tracers for tau, α-synuclein and TDP-43 proteinopathies will likely play a central role in further understanding pathophysiology associated with dementia spectrum disorders. Moreover, the development of reliable in vivo imaging tools could facilitate the development of new disease-modifying therapies and provide clinical diagnostic biomarkers.
References
Aarsland D, Beyer MK, Kurz MW (2008) Dementia in Parkinson’s disease. Curr Opin Neurol 21(6):676–682
Aarsland D et al (2017) Cognitive decline in Parkinson disease. Nat Rev Neurol 13(4):217–231
Aizenstein HJ et al (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65(11):1509–1517
Altmann A et al (2015) Regional brain hypometabolism is unrelated to regional amyloid plaque burden. Brain 138(Pt 12):3734–3746
Arbizu J et al (2013) Automated analysis of FDG PET as a tool for single-subject probabilistic prediction and detection of Alzheimer’s disease dementia. Eur J Nucl Med Mol Imaging 40(9):1394–1405
Arnold SE et al (1991) The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1(1):103–116
Arriagada PV et al (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42(3 Pt 1):631–639
Bagchi DP et al (2013) Binding of the radioligand SIL23 to alpha-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a Parkinson disease imaging agent. PLoS One 8(2):e55031
Barret O et al (2017) Initial clinical pet studies with the novel tau agent 18-F PI-2620 IN Alzheimer’s disease and controls. J Nucl Med 58(supplement 1):630
Bartus RT et al (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217(4558):408–414
Bateman RJ et al (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367(9):795–804
Bennett DA et al (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66(12):1837–1844
Benzinger TL et al (2013) Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci USA 110(47):E4502–E4509
Berger-Sweeney J (2003) The cholinergic basal forebrain system during development and its influence on cognitive processes: important questions and potential answers. Neurosci Biobehav Rev 27(4):401–411
Bevan Jones WR et al (2016) [18F]AV-1451 PET in behavioral variant frontotemporal dementia due to MAPT mutation. Ann Clin Transl Neurol 3(12):940–947
Bierer LM et al (1995a) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 52(1):81–88
Bierer LM et al (1995b) Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem 64(2):749–760
Birch AM, Katsouri L, Sastre M (2014) Modulation of inflammation in transgenic models of Alzheimer’s disease. J Neuroinflammation 11:25
Bohnen NI et al (2003) Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch Neurol 60(12):1745–1748
Bohnen NI et al (2005) Cognitive correlates of alterations in acetylcholinesterase in Alzheimer’s disease. Neurosci Lett 380(1–2):127–132
Bohnen NI et al (2006) Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol 253(2):242–247
Boutin H et al (2007) 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med 48(4):573–581
Brooks DJ (2009) Imaging amyloid in Parkinson’s disease dementia and dementia with Lewy bodies with positron emission tomography. Mov Disord 24(Suppl 2):S742–S747
Burre J et al (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329(5999):1663–1667
Cagnin A et al (2004) In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol 56(6):894–897
Cagnin A et al (2006) In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol Scand Suppl 185:107–114
Chauveau F et al (2009) Comparative evaluation of the translocator protein radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute neuroinflammation. J Nucl Med 50(3):468–476
Chien DT et al (2013) Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis 34(2):457–468
Cho H et al (2016) Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 87(4):375–383
Cho H et al (2017) 18F-AV-1451 binds to motor-related subcortical gray and white matter in corticobasal syndrome. Neurology 89(11):1170–1178
Clare R et al (2010) Synapse loss in dementias. J Neurosci Res 88(10):2083–2090
Cohen AD et al (2009) Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci 29(47):14770–14778
Collier TL et al (2017) cGMP production of the radiopharmaceutical [18 F]MK-6240 for PET imaging of human neurofibrillary tangles. J Labelled Comp Radiopharm 60(5):263–269
Compta Y et al (2014) The significance of alpha-synuclein, amyloid-beta and tau pathologies in Parkinson’s disease progression and related dementia. Neurodegener Dis 13(2–3):154–156
Crary JF et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128(6):755–766
Cselenyi Z et al (2012) Clinical validation of 18F-AZD4694, an amyloid-beta-specific PET radioligand. J Nucl Med 53(3):415–424
Davies CA et al (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci 78(2):151–164
De Vos H et al (1994) Imidazoline receptors, non-adrenergic idazoxan binding sites and alpha 2-adrenoceptors in the human central nervous system. Neuroscience 59(3):589–598
Declercq LD et al (2016) Drug development in Alzheimer’s disease: the contribution of PET and SPECT. Front Pharmacol 7:88
Delacourte A et al (2002) Tau aggregation in the hippocampal formation: an ageing or a pathological process? Exp Gerontol 37(10–11):1291–1296
Diehl J et al (2004) Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Aging 25(8):1051–1056
Diehl-Schmid J et al (2007) Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging 28(1):42–50
Doraiswamy PM et al (2014) Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry 19(9):1044–1051
Drzezga A et al (2008) Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage 39(2):619–633
Edison P et al (2007) Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68(7):501–508
Edison P et al (2008) Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis 32(3):412–419
Edison P et al (2013) Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology 38(6):938–949
Eggers C et al (2006) Cortical acetylcholine esterase activity and ApoE4 genotype in Alzheimer disease. Neurosci Lett 408:46–50
Engler H et al (2006) Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain 129(Pt 11):2856–2866
Engler H et al (2008) In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging 35(1):100–106
Fan Z et al (2015) Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 138(Pt 12):3685–3698
Fan Z et al (2016) Flutriciclamide (18F-GE180) PET: first-in-human PET study of novel third-generation in vivo marker of human translocator protein. J Nucl Med 57(11):1753–1759
Finnema SJ et al (2016) Imaging synaptic density in the living human brain. Sci Transl Med 8(348):348ra96
Fodero-Tavoletti MT et al (2009) In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur J Pharmacol 617(1–3):54–58
Fodero-Tavoletti MT et al (2011) 18F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 134(Pt 4):1089–1100
Fodero-Tavoletti MT et al (2012) In vitro characterization of [18F]-florbetaben, an Abeta imaging radiotracer. Nucl Med Biol 39(7):1042–1048
Fujishiro H et al (2010) Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett 486(1):19–23
Furst AJ et al (2012) Cognition, glucose metabolism and amyloid burden in Alzheimer’s disease. Neurobiol Aging 33(2):215–225
Garcia-Sevilla JA et al (1998) Imidazoline receptor proteins in brains of patients with Alzheimer’s disease. Neurosci Lett 247(2–3):95–98
Gilman S et al (2010) Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 74(18):1416–1423
Gomperts SN et al., Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol, 2016
Gomperts SN et al (2008) Imaging amyloid deposition in Lewy body diseases. Neurology 71(12):903–910
Gomperts SN et al (2012) Brain amyloid and cognition in Lewy body diseases. Mov Disord 27(8):965–973
Gomperts SM, Schultz A, Caso C (2015) [18F]T807 PET imaging of hyperphosphorylated tau to differentiate PSP from PD. In: Human amyloid imaging conference book of abstracts ID: 118
Gomperts SN et al (2016) PET radioligands reveal the basis of dementia in parkinson’s disease and dementia with Lewy bodies. Neurodegener Dis 16(1–2):118–124
Harada R et al (2015) [(18)F]THK-5117 PET for assessing neurofibrillary pathology in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 42(7):1052–1061
Harada R et al (2016) 18-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J Nucl Med 57(2):208–214
Hardy J (2006) Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis 9(3 Suppl):151–153
Head GA, Mayorov DN (2006) Imidazoline receptors, novel agents and therapeutic potential. Cardiovasc Hematol Agents Med Chem 4(1):17–32
Hilker R et al (2005) Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 65(11):1716–1722
Hirano S et al (2010) Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 133(Pt 7):2058–2068
Hirono N et al (2000) Features of regional cerebral glucose metabolism abnormality in corticobasal degeneration. Dement Geriatr Cogn Disord 11(3):139–146
Horti AG et al (2010) Development of radioligands with optimized imaging properties for quantification of nicotinic acetylcholine receptors by positron emission tomography. Life Sci 86:575–584
Horvath J et al (2013) Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Parkinsonism Relat Disord 19(10):864–868; (discussion 864)
Hosaka K et al (2002) Voxel-based comparison of regional cerebral glucose metabolism between PSP and corticobasal degeneration. J Neurol Sci 199(1–2):67–71
Hostetler ED et al (2016) Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J Nucl Med 57(10):1599–1606
Iannaccone S et al (2013) In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord 19(1):47–52
Ikawa M et al (2017) 11C-ER176, a radioligand for 18-kDa translocator protein, has adequate sensitivity to robustly image all three affinity genotypes in human brain. J Nucl Med 58(2):320–325
Ikonomovic MD et al (2008) Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 131(Pt 6):1630–1645
Imamura K et al (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106(6):518–526
Irie T et al (1994) Design and evaluation of radioactive acetylcholine analogs for mapping brain acetylcholinesterase (AchE) in vivo. Nucl Med Biol 21(6):801–808
Ishii K et al (1998a) Cerebral glucose metabolism in patients with frontotemporal dementia. J Nucl Med 39(11):1875–1878
Ishii K et al (1998b) Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology 51(1):125–130
Ishii K et al (2015) Regional glucose metabolic reduction in dementia with Lewy bodies is independent of amyloid deposition. Ann Nucl Med 29(1):78–83
Ittner LM, Gotz J (2011) Amyloid-beta and tau-a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12(2):65–72
Jack CR Jr et al (2010) Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 133(11):3336–3348
Jansen WJ et al (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama 313(19):1924–1938
Johnson KB, Sepulcre J, Rentz D (2014) Tau PET: initial experience with F18 T807. In: Human amyloid imaging conference book of abstracts ID: 103
Johnson KA et al (2016) Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 79(1):110–119
Jonasson M et al (2016) Tracer Kinetic analysis of (S)-(1)(8)F-THK5117 as a PET tracer for assessing tau pathology. J Nucl Med 57(4):574–581
Josephs KA et al (2016) [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132(6):931–933
Juh R et al., Different metabolic patterns analysis of Parkinsonism on the 18F-FDG PET. Eur J, Radiol (2004) 51(3): pp. 223–33
Juh R et al (2005) Cerebral glucose metabolism in corticobasal degeneration comparison with progressive supranuclear palsy using statistical mapping analysis. Neurosci Lett 383(1–2):22–27
Kaasinen V et al (2002) Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer’s disease. J Clin Psychopharmacol 22(6):615–620
Kadir A et al (2006) PET imaging of cortical 11C-nicotine binding correlates with the cognitive function of attention in Alzheimer’s disease. Psychopharmacology 188(4):509–520
Kadir A et al (2008) PET imaging of the in vivo brain acetylcholinesterase activity and nicotine binding in galantamine-treated patients with AD. Neurobiol Aging 29(8):1204–1217
Kantarci K et al (2012) Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging 33(9):2091–2105
Kantarci K et al (2017) AV-1451 tau and beta-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 81(1):58–67
Karran E, Hardy J (2014) A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol 76(2):185–205
Kealey S et al (2013) Imaging imidazoline-I2 binding sites in porcine brain using 11C-BU99008. J Nucl Med 54(1):139–144
Kennedy AM et al (1995) Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer’s disease. Neurosci Lett 186(1):17–20
Kertesz A et al (2005) The evolution and pathology of frontotemporal dementia. Brain 128(Pt 9):1996–2005
Kikuchi A et al., In vivo visualization of tau deposits in corticobasal syndrome by 18F-THK5351 PET. Neurology (2016) 87(22): p. 2309–2316
Kim EJ et al (2005) Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain 128(Pt 8):1790–1801
Klein RC et al (2005) Direct comparison between regional cerebral metabolism in progressive supranuclear palsy and Parkinson’s disease. Mov Disord 20(8):1021–1030
Knopman DS et al (2014) 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging 35(9):2096–2106
Kotagal V et al (2012) Thalamic cholinergic innervation is spared in Alzheimer disease compared to parkinsonian disorders. Neurosci Lett 514(2):169–172
Kreisl WC et al (2013) In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136(Pt 7):2228–2238
Kuhl DE et al (1996) In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol 40(3):399–410
Kuhl DE et al (1999) In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology 52(4):691–699
Landau SM et al (2011) Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 32(7):1207–1218
Langbaum JB et al (2010) Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol 67(4):462–468
Lant SB et al (2014) Patterns of microglial cell activation in frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 40(6):686–696
Leung K (2007) N-Acetyl-N-(2-[11C]methoxybenzyl)-2-phenoxy-5-pyridinamine. In: Molecular imaging and contrast agent database (MICAD). National Center for Biotechnology Information (US), Bethesda, MD; 2004–2013 [Updated 25 Aug 2011]
Li Y et al (2008) Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 35(12):2169–2181
Lim YY et al (2014) Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 137(Pt 1):221–231
Lohith T et al (2016) Preclinical evaluation and first-in-human dosimetry of [18F]MK-6240, a new PET tracer for in vivo quantification of human neurofibrillary tangles. J Nucl Med 57(supplement 2):125
Lohith T et al (2017) Quantification of [18F]MK-6240, a new PET tracer targeting human neurofibrillary tangles (NFTs) in brain of healthy elderly and subjects with Alzheimer’s disease. J Nucl Med 58(supplement 1):277
Lowe VJ et al (2014) Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology 82(22):1959–1967
Lowe VJ et al (2016) An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4(1):58
Maeda J et al (2004) Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: an imaging tool for glial cells in the brain. Synapse 52(4):283–291
Maetzler W et al (2008) [11C]PIB binding in Parkinson’s disease dementia. Neuroimage 39(3):1027–1033
Magistretti PJ (2000) Cellular bases of functional brain imaging: insights from neuron-glia metabolic coupling. Brain Res 886(1–2):108–112
Maragakis NJ, Rothstein JD (2001) Glutamate transporters in neurologic disease. Arch Neurol 58(3):365–370
Marquie M et al (2015) Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 78(5):787–800
Maruyama M et al (2013) Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79(6):1094–1108
Matsunaga S et al., Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol, 2015. 19(2)
Mazere J et al (2012) Progressive supranuclear palsy: in vivo SPECT imaging of presynaptic vesicular acetylcholine transporter with [123I]-iodobenzovesamicol. Radiology 265(2):537–543
McGeer PL et al (1993) Microglia in degenerative neurological disease. Glia 7(1):84–92
McKeith IG et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65(12):1863–1872
McKhann GM et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269
McMillan CT et al (2016) Multimodal evaluation demonstrates in vivo 18F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol 132(6):935–937
Mendez MF et al (2007) Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry 15(1):84–87
Miller ZA et al (2013) TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry 84(9):956–962
Minoshima S et al (1995) A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36(7):1238–1248
Minoshima S et al (2001) Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol 50(3):358–365
Mintun MD, Pontecorvo M, Joshi A, Potential for PET imaging tau tracer 18F-AV-1451 (also known as 18F-T807) to detect neurodegenerative progression in Alzheimer’s disease. Human amyloid imaging conference book of abstracts (2015) ID: 98
Mitsis EM et al (2009) 123I-5-IA-85380 SPECT imaging of nicotinic receptors in Alzheimer disease and mild cognitive impairment. J Nucl Med 50(9):1455–1463
Miyoshi M et al (2010) In vivo detection of neuropathologic changes in presymptomatic MAPT mutation carriers: a PET and MRI study. Parkinsonism Relat Disord 16(6):404–408
Moonga I et al (2017) Hypertension is associated with worse cognitive function and hippocampal hypometabolism in Alzheimer’s disease. Eur J Neurol 24(9):1173–1182
Morales I et al (2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8:112
Mueller A et al (2017) Characterization of the novel PET Tracer PI-2620 for the assessment of Tau pathology in Alzheimer’s disease and other tauopathies. J Nucl Med 58(supplement 1):847
Niccolini F, Politis M (2016) A systematic review of lessons learned from PET molecular imaging research in atypical parkinsonism. Eur J Nucl Med Mol Imaging 43(12):2244–2254
Nordberg A et al (1995) Kinetic analysis of regional (S)(-)11C-nicotine binding in normal and Alzheimer brains-in vivo assessment using positron emission tomography. Alzheimer Dis Assoc Disord 9(1):21–27
Nyback H et al (1994) PET studies of the uptake of (S)- and (R)-[11C]nicotine in the human brain: difficulties in visualizing specific receptor binding in vivo. Psychopharmacology 115:31–36
Okada H et al (2013) Alterations in alpha4beta2 nicotinic receptors in cognitive decline in Alzheimer’s aetiopathology. Brain 136(Pt 10):3004–3017
Okamura N, Yanai K (2010) Florbetapir (18F), a PET imaging agent that binds to amyloid plaques for the potential detection of Alzheimer’s disease. IDrugs 13(12):890–899
Okamura N et al (2005) Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer’s disease. J Neurosci 25(47):10857–10862
Okamura N et al (2014) Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain 137(Pt 6):1762–1771
Okello A et al (2009) Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 72(1):56–62
Olney NT, Spina S, Miller BL (2017) Frontotemporal Dementia Neurol Clin 35(2):339–374
Ossenkoppele R et al (2013) Differential effect of APOE genotype on amyloid load and glucose metabolism in AD dementia. Neurology 80(4):359–365
Ossenkoppele R et al (2015) Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. Jama 313(19):1939–1949
Ossenkoppele R et al (2016) Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139(Pt 5):1551–1567
Parker CA et al (2014) Evaluation of 11C-BU99008, a PET ligand for the imidazoline2 binding sites in rhesus brain. J Nucl Med 55(5):838–844
Passamonti L et al (2017) 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain 140(3):781–791
Perry EK et al (1977) Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J Neurol Sci 34(2):247–265
Petersen RC et al (2009) Mild cognitive impairment: 10 years later. Arch Neurol 66(12):1447–1455
Petit-Taboue MC et al (1991) Brain kinetics and specific binding of [11C]PK 11195 to omega 3 sites in baboons: positron emission tomography study. Eur J Pharmacol 200(2–3):347–351
Petrou M et al (2014) In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 55(3):396–404
Pike KE et al (2007) Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130(Pt 11):2837–2844
Politis M (2014) Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 10(12):708–722
Politis M, Piccini P (2012) Positron emission tomography imaging in neurological disorders. J Neurol 259(9):1769–1780
Politis M, Su P, Piccini P (2012) Imaging of microglia in patients with neurodegenerative disorders. Front Pharmacol 3:96
Politis M, Pagano G, Niccolini F (2017) Imaging in Parkinson’s disease. Int Rev Neurobiol 132:233–274
Poljansky S et al (2011) A visual [18F]FDG-PET rating scale for the differential diagnosis of frontotemporal lobar degeneration. Eur Arch Psychiatry Clin Neurosci 261(6):433–446
Pontecorvo MD, Joshi A, Lu M (2015) Relationships between florbetapir PET amyloid and 18F AV-1451 (aka 18F-T807) PET tau binding in cognitively normal subjects and patients with cognitive impairments suspected of Alzheimer’s disease. In: Human amyloid imaging conference book of abstracts ID: 98
Rabinovici GD, Miller BL (2010) Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs 24(5):375–398
Rabinovici GD et al (2007) 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 68(15):1205–1212
Rabinovici GD et al (2010) Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 133(Pt 2):512–528
Raboinovici GD et al (2015) Tau PET with [18F]AV1451 in non-alzheimer's disease neurodegenerative syndromes. Alzheimer's Dementia J Alzheimer's Assoc 11(7):P107–P109
Rayaprolu S et al (2013) TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener 8:19
Rocchi L, Niccolini F, Politis M (2015) Recent imaging advances in neurology. J Neurol 262(9):2182–2194
Roy R et al (2016) Cholinergic imaging in dementia spectrum disorders. Eur J Nucl Med Mol Imaging 43(7):1376–1386
Sabri O et al (2008) Acetylcholine receptors in dementia and mild cognitive impairment. Eur J Nucl Med Mol Imaging 35(Suppl 1):S30–S45
Sander K et al (2016) Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer’s disease, primary tauopathies, and other dementias. Alzheimers Dement 12(11):1116–1124
Schneider JA et al (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66(2):200–208
Schultz A et al., (2015) Relationship between longitudinal amyloid accumulation and T807-Tau, In: Human amyloid imaging conference book of abstracts, Submission ID 101. 2015:68
Schwarz AJ et al (2016) Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain 139(Pt 5):1539–1550
Serrano-Pozo A et al (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189
Shimada H et al (2009) Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 73(4):273–278
Shimada H et al (2013) beta-Amyloid in Lewy body disease is related to Alzheimer’s disease-like atrophy. Mov Disord 28(2):169–175
Shimada H, Shinotoh H, Sahara N (2015) Diagnostic utility and clinical significance of tau PET imaging with [11C]PBB3 in diverse tauopathies. In: Human amyloid imaging conference book of abstracts ID: 69
Shinotoh H et al (2004) Acetylcholinesterase imaging: its use in therapy evaluation and drug design. Curr Pharm Des 10(13):1505–1517
Silverman DH et al (2001) Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. Jama 286(17):2120–2127
Small GW, Kepe V, Barrio JR (2006) Seeing is believing: neuroimaging adds to our understanding of cerebral pathology. Curr Opin Psychiatry 19(6):564–569
Small GW et al (2008) Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol 7(2):161–172
Smith R et al (2017) In vivo retention of 18F-AV-1451 in corticobasal syndrome. Neurology 89(8):845–853
Spillantini MG et al (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840
Spina S et al (2017) Frontotemporal dementia with the V337M MAPT mutation: Tau-PET and pathology correlations. Neurology 88(8):758–766
Teipel S et al (2015) Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol 14(10):1037–1053
Terriere E et al (2010) 5-(123)I-A-85380 binding to the alpha4beta2-nicotinic receptor in mild cognitive impairment. Neurobiol Aging 31(11):1885–1893
Terry RD et al (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580
Tyacke RJ et al., Evaluation and initial in vitro and ex vivo characterization of the potential positron emission tomography ligand. BU99008 (2-(4,5-dihydro-1H-imidazol-2-yl)-1- methyl-1H-indole), for the imidazoline(2) binding site. Synapse, 2012. 66(6): 542–551
Vandenberghe R et al (2010) 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 68(3):319–329
Vander Borght T et al (1997) Cerebral metabolic differences in Parkinson’s and Alzheimer’s diseases matched for dementia severity. J Nucl Med 38(5):797–802
Villemagne VL et al (2014) In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging 41(5):816–826
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6(8):626–640
Walji AM et al (2016) Discovery of 6-(Fluoro-(18)F)-3-(1H-pyrrolo[2,3-c]pyridin-1-yl)isoquinolin-5-amine ([(18)F]-MK-6240): a positron emission tomography (PET) imaging agent for quantification of neurofibrillary tangles (NFTs). J Med Chem 59(10):4778–4789
Wang M, Gao M, Zheng QH (2012) Fully automated synthesis of PET TSPO radioligands [11C]DAA1106 and [18F]FEDAA1106. Appl Radiat Isot 70(6):965–973
Whitwell JL et al (2017) [18 F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord 32(1):124–133
Wiley CA et al (2009) Carbon 11-labeled pittsburgh compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol 66(1):60–67
Xia CF et al (2013) [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 9(6):666–676
Yasuno F et al (2008) Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry 64(10):835–841
Ye L et al (2008) In vitro high affinity alpha-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J Neurochem 105(4):1428–1437
Yong SW et al (2007) A comparison of cerebral glucose metabolism in Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies. Eur J Neurol 14(12):1357–1362
Yuan Y, Gu ZX, Wei WS (2009) Fluorodeoxyglucose-positron-emission tomography, single-photon emission tomography, and structural MR imaging for prediction of rapid conversion to Alzheimer disease in patients with mild cognitive impairment: a meta-analysis. AJNR Am J Neuroradiol 30(2):404–410
Zhang J (2015) Mapping neuroinflammation in frontotemporal dementia with molecular PET imaging. J Neuroinflammation 12:108
Zhang S et al (2012) Diagnostic accuracy of 18 F-FDG and 11 C-PIB-PET for prediction of short-term conversion to Alzheimer’s disease in subjects with mild cognitive impairment. Int J Clin Pract 66(2):185–198
Funding
Professor Marios Politis research is supported by Parkinson’s UK, Lily and Edmond J. Safra Foundation, Michael J Fox Foundation (MJFF) for Parkinson’s research, KCL’s NIHR Biomedical Research Unit, and the CHDI Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review boards and the local research ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained for all studies involving human participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wilson, H., Pagano, G. & Politis, M. Dementia spectrum disorders: lessons learnt from decades with PET research. J Neural Transm 126, 233–251 (2019). https://doi.org/10.1007/s00702-019-01975-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-01975-4