Abstract
Attention-deficit/hyperactivity disorder (ADHD) has been linked to dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, indexed by salivary cortisol. The phenotypic and aetiological association of cortisol productivity with ADHD was investigated. A selected twin design using 68 male twin-pairs aged 12–15, concordant or discordant for high ADHD symptom scores, or control twin-pairs with low ADHD symptoms, based on developmentally stable parental ADHD ratings. A genetic growth curve model was applied to cortisol samples obtained across three points during a cognitive-electroencephalography assessment, to examine the aetiological overlap of ADHD affection status (high versus low ADHD symptom scores) with latent intercept and slope factors. A significant phenotypic correlation emerged between ADHD and the slope factor, with cortisol levels dropping faster for the group with high ADHD symptom scores. The analyses further suggested this overlap was mostly driven by correlated genetic effects. We identified change in cortisol activity over time as significantly associated with ADHD affection status, primarily explained by shared genetic effects, suggesting that blunted cortisol productivity can be a marker of genetic risk in ADHD.
Similar content being viewed by others
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterised by age-inappropriate levels of inattention and hyperactivity-impulsivity. ADHD symptoms have a strong genetic component, with heritability estimated at around 70 % (Burt 2009; Faraone et al. 2005; Wood et al. 2010b). The investigation of objectively measured physiological and neurobiological measures as potential biological correlates of the ADHD phenotypes may capture underlying processes that lie closer to ADHD genetic liability and delineate pathways from genes to ADHD behaviours.
Studies on children with diagnosed ADHD versus control groups examining overall diurnal cortisol levels and rhythm have reported significantly lower basal cortisol concentrations in morning sampling (Blomqvist et al. 2007; Isaksson et al. 2012; Ma et al. 2011), and significantly lower incidence of typical diurnal variation (defined as having a maximum and minimum cortisol level, respectively, at morning and evening sampling (Kaneko et al. 1993). Other studies on stress reactivity have reported increased cortisol productivity (White and Mulligan 2005), and conversely significantly lower pre- and post-stressor (IV insertion) cortisol concentrations (McCarthy et al. 2011) in children with ADHD, and blunted cortisol reactivity linked to persistent ADHD (King et al. 1998). In addition, one study reported a significantly higher incidence of atypical cortisol non-suppression to dexamethasone in ADHD versus control group (Kaneko et al. 1993). Overall, a recent meta-analysis of case–control studies of baseline cortisol found a significant, though modest effect (d = −0.31, p = 0.0001) of ADHD, with lower levels in ADHD versus controls (Scassellati et al. 2012). Some studies suggest that the covariation between ADHD and HPA-axis dysregulation is further complicated by ADHD subtype and/or comorbidity (Freitag et al. 2009; Hastings et al. 2009), but in the largest study to date with over 300 children (Isaksson et al. 2012), the negative association of morning cortisol measures with ADHD symptoms in children aged over 10 years did not differ according to ADHD subtype or co-occurring symptoms.
The heritability of HPA axis functioning has been investigated in twin studies. A review of twin studies (Bartels et al. 2003a) reported substantial variation in heritability estimates for basal cortisol measures (0–84 %), likely reflective of varying methodologies. Moreover, the authors found that none of the reviewed studies had adequate sample sizes to disentangle familial covariation into genetic versus shared environmental components. Pooling together five comparable twin studies yielded a heritability estimate of 62 % (Bartels et al. 2003a). In a subsequent study conducted on cortisol sampled over two consecutive days in 180 children aged 12, genetic factors were negligible for evening samples, with moderate heritability for cortisol sampled at awakening and before lunch, and highest (56–59 %) for samples collected up to an hour after awakening (Bartels et al. 2003b) A study, based on a subsample of same-sex twins selected from the Twins’ Early Development Study (TEDS; where the current sample is also derived from), found substantial heritability for single-point measures (around 60 %; pre-task baseline and two post-task values) and more moderate heritability for cortisol reactivity (44 %) to a computerised behavioural challenge (Steptoe et al. 2009).
The heritability of ADHD symptoms is comparatively well established (Burt 2009; Faraone et al. 2005; Wood et al. 2010a, b). Moreover, a series of studies on the TEDS sample suggest that stability in ADHD symptomatology is largely attributed to genetic effects (Kuntsi et al. 2005) and that the two ADHD symptom domains show substantial genetic overlap, as well as some degree of genetic distinction (Greven et al. 2011; McLoughlin et al. 2007). Furthermore, twin analyses indicate that, despite rater-specific effects, informant ratings largely index the same genetically influenced behaviours (Merwood et al. 2013).
However, the extent to which the genetic influences on cortisol measures are shared with genetic influences on ADHD has not yet been addressed and is a key objective of this study. If shared genetic risk is demonstrated, then indices of HPA-axis activity could be a biomarker for ADHD. Furthermore, if overlapping genetic effects are identified and can be distinguished as representing mediating genetic effects [as opposed to representing pleiotropic (alternative manifestations of the same genetic risk factors) genetic effects], then cortisol dysregulation can be identified as reflecting a causal pathway underlying ADHD and indicate a potential target for treatment and preventative interventions. Finally, the low levels of cortisol productivity may be related to under-arousal which has been theorised as underlying ADHD symptomatology [e.g. the state-regulatory (van der Meer, 2002) and the arousal-attention (Johnson et al. 2007; O’Connell et al. 2008) model].
This study investigated the association between ADHD affection status and cortisol activity in a sample of male adolescent twin pairs selected from a population-based sample according to consistent high and low ADHD symptoms (McLoughlin et al. 2014; Tye et al. 2012). Specifically, we aimed to explore the aetiological relationship between ADHD affection status (high versus low ADHD symptom scores) and the dynamics of cortisol productivity as captured by the intercept and slope variables of a linear growth curve model (GCM), while also modelling potential covariate effects (e.g. age, sampling conditions and demographic factors) on the means of these latent variables. We hypothesised that ADHD affection status would be associated with atypical cortisol productivity, specifically blunted cortisol activity for the group with high ADHD symptom scores. We had no a priori hypotheses regarding the aetiological relationship of ADHD affection status and cortisol activity.
Methodology
Sample
The sample was selected from TEDS, a birth cohort study, which invited all twins born in England and Wales between 1994 and 1996 to enrol (Trouton et al. 2002). Zygosity was determined using a zygosity questionnaire that has been shown to have 95 % accuracy (Price et al. 2000). Where zygosity was unclear from this questionnaire, DNA testing was conducted. The TEDS sample is representative of the general population in terms of parental education, ethnicity and employment status (Haworth et al. 2013).
The Neurophysiological study of Activity and Attention in Twins (NEAAT) subset used in this study consisted of 68 male twin pairs aged between 12 and 15. Twin pairs were selected based on latent class trajectory analysis of ADHD symptoms at ages 8, 12 and 14, using the 18 DSM-IV based ADHD items from the Conners’ Parent Rating Scale (Conners et al. 1998). This approach identified sub-groups of individuals who have been consistently rated by parents as having high or low ADHD symptoms and thus ensured selection of twin pairs consistently concordant or discordant for high levels of ADHD symptoms or unaffected controls (consistently low ADHD symptom ratings). Further information on selection of twins is given elsewhere (see McLoughlin et al. (2014) and Tye et al. (2012) for supplementary information). The selected sample consisted of 22 pairs concordant for high ADHD symptoms (11 monozygotic (MZ) and 11 dizygotic (DZ) pairs), 8 pairs discordant for ADHD symptoms (2 MZ and 6 DZ pairs) and 38 control pairs concordant for low ADHD symptoms (22 MZ and 16 DZ pairs). When subdividing our sample according to ADHD symptom ratings, 84 participants were classified as controls (low ADHD symptom scores) and 52 participants placed in the ‘high ADHD symptoms’ group.
Participating families gave their written informed consent and the study was approved by King’s College London Psychiatry, Nursing and Midwifery Research Ethics Sub-Committee (PNM/08/09-089).
Measures
Salivary cortisol
Salivary cortisol was obtained using the passive drool method at three time points during the cognitive-electroencephalography (EEG) session. The baseline measure was collected approximately 40 min after entering the EEG lab. Prior to baseline measurement, the child watched a cartoon while the EEG cap and electrodes were prepared for data collection and had two resting EEG periods (eyes open and closed; 3 min each). The child then completed three cognitive tasks while EEG was simultaneously measured (described below). On average, cortisol levels peak 20 min after stressor onset (Hirvikoski et al. 2009) and, therefore, to index participants’ cortisol response to the second cognitive task, the second sampling of saliva was obtained at the end of the last cognitive task (approximately an hour after baseline measurement). The final salivary sample was collected approximately 20 min later, to index response to the third cognitive task.
11 individuals (8 controls and 3 cases) were unable or did not agree to participate in saliva collection during the cognitive-EEG testing session. There were no significant differences in age or ADHD group status between those who did or did not participate. On the day of laboratory testing, four children had taken over-the-counter medication (e.g. pain killers) and an additional five children had taken steroid-based asthma inhalers. Two dichotomous variables were created [steroid-based medication (yes/no), and other medication (yes/no)]. There were no significant differences according to medication status for either variable, and, therefore, all participants’ samples were retained for analysis.
All uncentrifuged saliva samples were stored in a −80 °C medical freezer, until completion of the study when they were assayed for cortisol. Salivary samples from 10 participants were sent for preliminary analysis, with all remaining samples analysed in a single batch using a high-sensitivity chemiluminscence assay (Salimetrics, Cambridge, UK). The lower limit of detectable sensitivity was <0.003 µg/dL (micrograms per decilitre). One of the 375 analysed samples had insufficient saliva volume to test for cortisol. Coefficients of variance were below 10 % and, therefore, not subjected to re-testing. Cortisol concentrations were provided in µg/dL and converted into nmol/l (nanomoles per litre) by multiplying original values by 27.59.
Cortisol values were screened for extreme values. All samples from one control participant were higher than three standard deviations (SD) from the mean and designated as missing. Another control participant had one outlier (defined as a value >3 SD above the mean) and was winsorized (re-assigned with the highest individual corresponding value <3 SD above the mean).
Cognitive-EEG tasks
Task 1: cued continuous performance test (CPT-OX) with flankers 33 (11 min) (Doehnert et al. 2008; Valko et al. 2009). This well-validated cued CPT involves selective attention, preparation and vigilance for an infrequently occurring stimulus, plus an embedded go/no-go test. The stimuli consist of a black letter array formed of a centre letter flanked on each side by distractor letters. Participants are instructed to respond only to cue-target sequences (XOX-OXO), by pressing a button as quickly as possible with the index finger of their preferred hand.
Task 2: arrow flanker task (13 min) (Albrecht et al. 2005; McLoughlin et al. 2009, 2014). This standard arrow flanker task is designed to measure brain activity during conflict monitoring. Two flankers (black arrowheads above and below the position of a fixation mark) are first presented 100 ms before the central target black arrowhead appears. Participants have to press a response button corresponding to the direction indicated by the target arrow. On congruent trials, flanker and target arrowheads point in the same direction; on incongruent trials, they point in opposite directions. If the participants are being too careful and not making any errors, they are instructed to perform at a faster rate, with the intention that this will cause them to make errors. Conversely, if the participant is making too many errors, they are given feedback to be more careful in their responses.
Task 3: the fast task (20 min) (Andreou et al. 2007; Kuntsi et al. 2006). This four-choice reaction time task measures performance under baseline (slow, unrewarded) and fast-incentive conditions. Four empty circles first appear on the screen. After one of them (the target) is coloured in, participants have to press an appropriate response key as quickly as possible. In the fast-incentive condition the participants win a smiley face whenever responding faster than their own mean reaction time (MRT) during the baseline condition consecutively for three trials.
ADHD rating scales
ADHD ratings scores were derived from the 18-item DSM-IV based ADHD subscale of the Long Version of the Conners’ Parent Revised Rating Scale (Conners et al. 1998).
Statistical analysis
Genetic growth curve model
The relationship between ADHD affection status and cortisol measures taken during the cognitive-EEG session was tested in a linear growth curve model (GCM) which captures the dynamics of the cortisol activity (an index of HPA axis function) by means of two latent factors: the intercept [cortisol concentration at the beginning of the testing paradigm (I)] and the slope [rate of change in cortisol productivity over time (S)]. The loadings for the intercept were fixed at 1 and those of the slope to 0, 1 and 2 to reflect the linear trajectory of cortisol over the testing period (Fig. 1). The GCM captures each individual’s trajectory across all sampling points, rather than focussing on individual measures (Hagger-Johnson et al. 2010) and, therefore, does not require complete data for every participant and estimates both parameters simultaneously within the same model.
The genetic nature of the twin sample further allowed estimation of the aetiological variance and covariance of these latent intercept and slope factors as well as ADHD affection status according to general biometrical genetic theory. In brief, twin studies enable us to disentangle the extent to which a trait is influenced by genetic factors (A), shared environmental factors (C), and non-shared environmental influences (E). This method relies on the difference in genetic relatedness between MZ twins, who share all of their genetic information, and DZ twins who share, on average, only 50 % of their additive genes. Multivariate genetic models estimate the underlying variance components of individual variables (or latent factors) and the aetiological components of phenotypic covariance between multiple traits (or factors) (Neale and Cardon 1992).
Main effects of covariates like age and sampling conditions were specified on the intercept and slope factor means while modelling the genetic architecture of these factors. The genetic and environmental variance and covariance parameters of the continuous cortisol measures and ordinal affection status were estimated using structural equation modelling in the OpenMx program (Boker et al. 2011). Using full information maximum likelihood estimation, parameters were obtained in a joint continuous-ordinal model in which a liability threshold model was assumed to express the risk for ADHD as normally distributed in the general population with the disorder occurring when a certain threshold at symptoms’ level is exceeded. However, to get unbiased estimates for the association with the cortisol factors, the non-random selection of individuals on ADHD required all its parameters to be fixed to population known values: h2 = 0.76, c2 = 0, e2 = 0.24 (derived from the mean heritability pooled across over 20 twin studies (Faraone et al. 2005); and the threshold to a lifetime risk of 5 % (Polanczyk et al. 2007). This model has been applied to schizophrenia and bipolar disorder in analyses on brain volumes (Rijsdijk et al. 2005) and neuropsychological measures (Toulopoulou et al. 2007), as well as on electrophysiological parameters on the same sample as reported here (McLoughlin et al. 2014; Tye et al. 2012).
Figure 2 presents the correlated factors solution of the fitted genetic Cholesky model on intercept, slope and ADHD factors, where the ordering of variables is arbitrary (Loehlin 1996). It gives the genetic (rA), shared environmental (rC) and nonshared environmental (rE) correlations (i.e. the extent to which the same genetic or environmental factors contribute to trait covariation). In addition, using the standardized path estimates, we calculated the proportion of the phenotypic overlap between slope and ADHD due to genetic effects (rph-A = √h 2 Slope × rA × √0.76) and the unique environmental effects (rph-E = √e 2 Slope × rE × √0.24) (for more details see Neale and Cardon 1992; Rijsdijk et al. 2005).
Genetic GCM for cortisol sampling during cognitive EEG-testing paradigm and ADHD affection status, with age effects incorporated in Intercept and slope factor means. LAB_1 baseline, LAB_2 sampled at end of cognitive tasks, LAB_3 sampled at end of cognitive-EEG assessment, As ADHD affection status; I intercept, S slope, Esp residual measurement error, µ i intercept mean; µ s slope mean, μ io overall mean of the intercept factor, μ so overall mean of the slope factor, β i age-dependent intercept mean, β s age-dependent slope mean; the variances of the intercept and slope factors are 0.26 and 0.03, respectively; model presented for one twin only for ease of presentation
Results
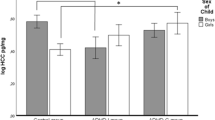
As expected, the ADHD group had significantly higher mean ADHD DSM-IV based symptom scores (Table 1), confirming that the two groups significantly differed according to key ADHD diagnostic criteria. Other group mean differences include that the group with high ADHD symptom scores was significantly younger than controls and were more likely to have participated during months with more light (March–September). In addition, on the day of the cognitive-EEG session, the high ADHD symptom group had significantly later awakening and later sampling times. The high ADHD symptom group had lower cortisol concentration levels throughout the cognitive-EEG session, although this was only significant at the last sampling point and did not retain significance (t = −1.34, p = 0.18) after regressing out potential confounders (age, awakening time, sampling time, and season of sampling).
The aetiological relationship between ADHD and cortisol activity was examined by testing the overlap with the intercept and slope factors, respectively, as measured in a latent growth factor model. We ran separate models controlling individually for potential confounding effects of all the sampling factors (age, awakening time, sampling time and season of sampling) on the latent factor means (mI = 1.31 and mS = −0.25). The only significant effect was found for age on the intercept (β I age = 0.24). Accordingly, only age was incorporated in the final model (Fig. 2). Familial factors largely explained individual differences on both the intercept [h 2 = 46 % (95 % CI 0/97 %), c 2 = 29 % (0/83 %) and e 2 = 25 % (3/66 %)] and the slope factor [h 2 = 21 % (0/99 %), c 2 = 64 % (0/98 %) and e 2 = 15 % (0/99 %)]. ADHD only significantly correlated with the slope factor [−0.49 (−0.98/−0.09)], implicating that cortisol concentrations decreased significantly faster for the affected group. The phenotypic overlap between the slope factor and ADHD affection status due to genetic effects (rph-A = √0.21 × −0.89 × √0.76) was −0.36 and that due to unique environmental effects (rph-E = √0.15 × −0.71 × √0.24) was -0.13. This means that the phenotypic overlap between cortisol change over time and ADHD status was predominantly (−0.36/−0.49 = 73 %) due to shared genetic factors (within the limitations of the fixed model which assumes no effects of C on ADHD affection status). The standardized estimates for the individual cortisol measures are presented in Table 2. Heritability estimates for the individual cortisol measures ranged from 22 to 33 %, but were not detectable as significant in this small sample. Estimates for shared and unique environmental components were largely similar across the individual cortisol measures. Unique environmental variance contributed to the growth factors, as well as significant residual variance (which includes measurement error).
Discussion
In an analysis of adolescent twin pairs selected for developmentally stable high vs low ADHD symptoms, two key findings emerged that highlight the dynamics of cortisol productivity and its aetiological link to ADHD. First, while we did not find the expected group differences in all single-point measures across the trajectory of cortisol concentrations over laboratory testing, we found, using growth curve modelling (GCM), that cortisol levels declined at a faster rate for the high ADHD symptom group, with a significantly steeper slope, compared to controls. In contrast, the intercept was similar across groups. Our second main finding was that the significant overlap between ADHD and the slope factor was predominantly due to shared genetic factors.
Taken together, our findings suggest that significant group differences relate to dynamic (degree of change) indicators of HPA functioning during cognitive-EEG testing, rather than baseline levels. A methodological implication of our findings is that GCM represents an effective alternative means of examining cortisol activity over a given period of time, in line with increasing research that suggests that patterns of activity are generally not adequately captured by single-point or absolute-difference measures (Balodis et al. 2010).
Further, the rate of cortisol change in productivity, as indexed by the slope, captures a majority of the genetic risk in ADHD. The shared genetic risk is an important finding and suggests that cortisol dysregulation is a candidate endophenotype for the ADHD. It may be that blunted cortisol activity is a biological marker of the ADHD phenotype and a potential neurobiological pathway underlying ADHD symptomatology. In particular, if future studies can clarify if the overlapping genetic effects represent causal pathways (as opposed to pleiotropy), then indices of HPA-axis functioning may be identified as a potential target for treatment and preventative interventions. In relation to theoretical models of ADHD, attenuated cortisol levels have been suggested to reflect under-arousal, in line with arousal dysregulation models (Isaksson et al. 2012), such as the state-regulatory model (van der Meer 2002) or the arousal-attention model (Johnson et al. 2007; O’Connell et al. 2008). Our findings add to the previous findings by specifying the dynamics of cortisol productivity as a key process in ADHD, which future studies can examine in relation to cognitive-EEG and skin conductance measures of the proposed arousal dysregulation in ADHD.
It is important to emphasise that the current study on cognitive-EEG testing was part of a larger battery of assessment and was not intended to provoke major stress. Yet, across both groups the first sample yielded the highest cortisol concentrations (Table 1; Fig. 1), suggesting potentially anticipatory stress and HPA activation prior to entering the EEG lab. It is possible that the pattern of activity captured is reflecting only part of the cortisol-reactivity curve: subsequent recovery from the anticipation of entering the laboratory. Repeated sampling over a longer sampling time would allow a more detailed examination of the cortisol-reactivity pattern.
It is important to note that the cortisol concentrations sampled were skewed to the right and that maximum-likelihood statistical equational modelling requires data to be normally distributed. However, as the means and variances of the GCM factors rely on changes in means of the observed variables, any transformations that would change the natural order of the means (e.g. log transformation) would render the variables unsuitable for GCM. This is a limitation of the current analyses and could potentially be avoided using multiple threshold models, albeit with a severe loss of statistical power (Mehta et al. 2004). In addition, we were unable to distinguish between ADHD behavioural dimensions in our study, as children were selected for consistently high/low parental ratings across both symptom domains; yet the evidence from other studies suggests that blunted cortisol activity in ADHD may have a stronger relationship to hyperactivity-impulsivity, as opposed to inattention (Ma et al. 2011; Maldonado et al. 2009). Along the same lines, it was not possible to model any moderation (or main) effects of co-occurring behaviours, such as oppositional behaviours or anxiety-shy symptoms, on ADHD liability and the relationship with the slope factor, given the selected nature of the sample and the fixed parameters on ADHD to correct for selection. Although some studies have suggested that the covariation between ADHD and HPA-axis dysfunctioning may be complicated by comorbidity (particularly comorbid ODD), our previous twin analyses focusing on middle childhood suggested that hyperactivity-impulsivity and oppositional behaviours largely index the same aetiological liability (Wood et al. 2009), raising the possibility that associations with ADHD (particularly hyperactivity-impulsivity) and/or ODD may be capturing the same effect.
One of the main strengths of this study was that the sample was limited to include males only and to a restricted age range, to reduce variability and maximize sample homogeneity, although an important caveat is that we did not have information relating to pubertal status, which may be a significant source of variance in cortisol productivity. This study relied on parental ratings of ADHD, as opposed to a diagnosis made within a clinical setting, and so avoids biases associated with referral bias, comorbidity and the confounding effects of stimulant medication. Although this may limit the generalizability of our findings to non-population based studies, the overall evidence from family and twin studies strongly suggest that the clinical diagnosis of ADHD reflects the extreme and impairing tail end of one or more continuous dimensions of psychopathology (Chen et al. 2008; Levy et al. 1997). Moreover, a series of analyses have highlighted the comparability of our quantitative genetic findings across both diagnostic and dimensional approaches to the ADHD phenotype (Cheung et al. 2012, 2014; Kuntsi et al. 2010, 2014; Paloyelis et al. 2010; Wood et al. 2010a, 2011). Although our sample size was small, when studying low prevalence disorders in twin model fitting power can be increased by using a selected sample (Neale et al. 1994), as utilised in our study.
In conclusion, in a novel application of growth curve modelling (GCM) and the twin design to examine cortisol activity with ADHD affection status, we found that ADHD is specifically associated with the dynamics of cortisol productivity and that this overlap is predominantly due to shared genetic factors. Future research incorporating genotype, cognitive-EEG and skin conductance data will be able to further delineate the affected pathways. Increased awareness of the pathophysiological processes involved in ADHD may facilitate improvements in treatment and management of the disorder (Corominas et al. 2012).
References
Albrecht B, Banaschewski T, Brandeis D, Heinrich H, Rothenberger A (2005) Response inhibition deficits in externalizing child psychiatric disorders: an ERP-study with the stop-task. Behav Brain Funct 1:1–22
Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Meidad S, Muller UC, Uebel H, Banaschewski T, Manor I, Oades R, Roeyers H, Rothenberger A, Sham P, Steinhausen HC, Asherson P, Kuntsi J (2007) Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med 37:1703–1715
Balodis IM, Wynne-Edwards KE, Olmstead MC (2010) The other side of the curve: examining the relationship between pre-stressor physiological responses and stress reactivity. Psychoneuroendocrinology 35:1363–1373
Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJ (2003a) Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology 28:121–137
Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI (2003b) Heritability of daytime cortisol levels in children. Behav Genet 33:421–433
Blomqvist M, Holmberg K, Lindblad F, Fernell E, Ek U, Dahllof G (2007) Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur J Oral Sci 115(1):1–6
Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabook R, Kenny S, Bates T, Mehta P, Fox J (2011) OpenMx: an open source extended structural equation modeling framework. Psychometrika 76:306–317
Burt SA (2009) Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychol Bull 135:608–637
Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, Fleischman K, Knight J, Andreou P, Arnold R, Altink M, Boer F, Boholst MJ, Buschgens C, Butler L, Christiansen H, Fliers E, Howe-Forbes R, Gabriels I, Heise A, Korn-Lubetzki I, Marco R, Medad S, Minderaa R, Muller UC, Mulligan A, Psychogiou L, Rommelse N, Sethna V, Uebel H, McGuffin P, Plomin R, Banaschewski T, Buitelaar J, Ebstein R, Eisenberg J, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P (2008) DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet 147B:1450–1460
Cheung CHM, Wood AC, Paloyelis Y, Arias-Vasquez A, Buitelaar JK, Franke B, Miranda A, Mulas F, Rommelse N, Sergeant JA, Sonuga-Barke EJS, Faraone SV, Asherson P, Kuntsi J (2012) Aetiology for the covariation between combined type ADHD and reading difficulties in a family study: the role of IQ. J Child Psychol Psychiatry 53:864–873
Cheung CHM, Fazier-Wood AC, Asherson P, Rijsdijk F, Kuntsi J (2014) Shared cognitive impairments and aetiology in ADHD symptoms and reading difficulties. PLoS One 9:e98590
Conners CK, Sitarenios G, Parker JD, Epstein JN (1998) The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268
Corominas M, Ramos-Quiroga JA, Ferrer M, Saez-Francas N, Palomar G, Bosch R, Casas M (2012) Cortisol responses in children and adults with attention deficit hyperactivity disorder (ADHD): a possible marker of inhibition deficits. Atten Defic Hyperact Disord 4:63–75
Doehnert M, Brandeis D, Straub M, Steinhausen HC, Drechsler R (2008) Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: is there neurophysiological evidence for specific effects? J Neural Transm 115:1445–1456
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005) Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1313–1323
Freitag CM, Hanig S, Palmason H, Meyer J, Wust S, Seitz C (2009) Cortisol awakening response in healthy children and children with ADHD: impact of comorbid disorders and psychosocial risk factors. Psychoneuroendocrinology 34:1019–1028
Greven CU, Rijsdijk FV, Plomin R (2011) A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. J Abnorm Child Psychol 39:265–275
Hagger-Johnson GE, Whiteman MC, Wawrzyniak AJ, Holroyd WG (2010) The SF-36 component summary scales and the daytime diurnal cortisol profile. Qual Life Res 19:643–651
Hastings PD, Fortier I, Utendale WT, Simard LR, Robaey P (2009) Adrenocortical functioning in boys with attention-deficit/hyperactivity disorder: examining subtypes of ADHD and associated comorbid conditions. J Abnorm Child Psychol 37:565–578
Haworth CM, Davis OS, Plomin R (2013) Twins Early Development Study (TEDS): a genetically sensitive investigation of cognitive and behavioral development from childhood to young adulthood. Twin Res Hum Genet 16:117–125
Hirvikoski T, Lindholm T, Nordenstrom A, Nordstrom AL, Lajic S (2009) High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Horm Behav 55:418–424
Isaksson J, Nilsson KW, Nyberg F, Hogmark A, Lindblad F (2012) Cortisol levels in children with attention-deficit/hyperactivity disorder. J Psychiatr Res 46:1398–1405
Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, Robertson IH (2007) Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia 45:630–638
Kaneko M, Hoshino Y, Hashimoto S, Okano T, Kumashiro H (1993) Hypothalamic-pituitary-adrenal axis function in children with attention-deficit hyperactivity disorder. J Autism Dev Disord 23:59–65
King JA, Barkley RA, Barrett S (1998) Attention-deficit hyperactivity disorder and the stress response. Biol Psychiatry 44:72–74
Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R (2005) Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biol Psychiatry 57:647–654
Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere J, Rijsdijk F, Asherson P (2006) Reaction time, inhibition, working memory and ‘delay aversion’ performance: genetic influences and their interpretation. Psychol Med 36:1613–1624
Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NNJ, Sergeant JA, Sonuga-Barker EJ, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen H-C, Faraone SV, Asherson P (2010) Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry 67:1159–1167
Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, Asherson P (2014) The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. J Abnorm Child Psyol 42:127–136
Levy F, Hay DA, McStephen M, Wood C, Waldman I (1997) Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry 36:737–744
Loehlin JC (1996) The Cholesky approach: a cautionary note. Behav Genet 26:65–69
Ma L, Chen YH, Chen H, Liu YY, Wang YX (2011) The function of hypothalamus-pituitary-adrenal axis in children with ADHD. Brain Res 1368:159–162
Maldonado EF, Trianes MV, Cortes A, Moreno E, Escobar M (2009) Salivary cortisol response to a psychosocial stressor on children diagnosed with attention-deficit/hyperactivity disorder: differences between diagnostic subtypes. Span J Psychol 12:707–714
McCarthy AM, Hanrahan K, Scott LM, Zemblidge N, Kleiber C, Zimmerman MB (2011) Salivary cortisol responsivity to an intravenous catheter insertion in children with attention-deficit/hyperactivity disorder. J Pediatr Psychol 36:902–910
McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R (2007) Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. J Abnorm Child Psychol 35:999–1008
McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J (2009) Performance monitoring is altered in adult ADHD: a familial event-related potential investigation. Neuropsychologia 47:3134–3142
McLoughlin G, Palmer JA, Rijsdijk F, Makeig S (2014) Genetic overlap between evoked frontocentral theta-band phase variability, reaction time variability, and attention-deficit/hyperactivity disorder symptoms in a twin study. Biol Psychiatry 75:238–247
Mehta PD, Neale MC, Flay BR (2004) Squeezing interval change from ordinal panel data: latent growth curves with ordinal outcomes. Psychol Methods 9:301–333
Merwood A, Greven CU, Price TS, Rijsdijk F, Kuntsi J, McLoughlin G, Larsson H, Asherson PJ (2013) Different heritabilities but shared etiological influences for parent, teacher and self-ratings of ADHD symptoms: an adolescent twin study. Psychol Med 43:1973–1984
Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Kluwer, Dordrech (Netherlands)
Neale MC, Eaves LJ, Kendler KS (1994) The power of the classical twin study to resolve variation in threshold traits. Behav Genet 24:239–258
O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Fitzgerald M, Robertson IH (2008) Self-Alert Training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia 46:1379–1390
Paloyelis Y, Rijsdijk F, Wood AC, Asherson P, Kuntsi J (2010) The genetic association between ADHD symptoms and reading difficulties: the role of inattentiveness and IQ. J Abnorm Child Psyol 38:1083–1095
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948
Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R (2000) Infant zygosity can be assigned by parental report questionnaire data. Twin Res 3:129–133
Rijsdijk FV, van Haren NE, Picchioni MM, McDonald C, Toulopoulou T, Hulshoff Pol HE, Kahn RS, Murray R, Sham PC (2005) Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med 35:1399–1409
Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (2012) Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatry 51:1003–1019
Steptoe A, van Jaarsveld CH, Semmler C, Plomin R, Wardle J (2009) Heritability of daytime cortisol levels and cortisol reactivity in children. Psychoneuroendocrinology 34:273–280
Toulopoulou T, Picchioni M, Rijsdijk F, Hua-Hall M, Ettinger U, Sham P, Murray R (2007) Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch Gen Psychiatry 64:1348–1355
Trouton A, Spinath FM, Plomin R (2002) Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res 5:444–448
Tye C, Rijsdijk F, Greven CU, Kuntsi J, Asherson P, McLoughlin G (2012) Shared genetic influences on ADHD symptoms and very low-frequency EEG activity: a twin study. J Child Psychol Psychiatry 53:706–715
Valko L, Doehnert M, Muller UC, Schneider G, Albrecht B, Drechsler R, Maechler M, Steinhausen HC, Brandeis D (2009) Differences in neurophysiological markers of inhibitory and temporal processing deficits in children and adults with ADHD. J Psychophysiol 23:235–246
van der Meer JJ (2002) Hyperactivity and attention disorders of childhood. In: Sandberg S (ed) Title, 2nd edn. Cambridge University Press, Cambridge, pp 162–213
White BP, Mulligan SE (2005) Behavioral and physiologic response measures of occupational task performance: a preliminary comparison between typical children and children with attention disorder. Am J Occup Ther 59:426–436
Wood AC, Rijsdijk F, Asherson P, Kuntsi J (2009) Hyperactive-impulsive symptom scores and oppositional behaviours reflect alternate manifestations of a single liability. Behav Genet 39:447–460
Wood AC, Asherson P, van der Meere JJ, Kuntsi J (2010a) Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on IQ. Psychol Med 40:1027–1037
Wood AC, Buitelaar J, Rijsdijk F, Asherson P, Kuntsi J (2010b) Rethinking shared environment as a source of variance underlying attention-deficit/hyperactivity disorder symptoms: comment on Burt (2009). Psychological Bull 136:331–340
Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, Arias-Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NNJ, Sergeant JA, Sonuga-Bark EJS, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Mano I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV, Asherson P, Kuntsi J (2011) The relationship between ADHD and key cognitive phenotypes is not mediated by shared familial effects with IQ. Psychol Med 41:861–871
Acknowledgments
The authors gratefully acknowledge the participating families and all staff involved in this study, in particular, Charlotte Tye, Chloe Booth, Sarah Lewis, Stuart Newman, the Twins’ Early Development Study research team, and the Director of the Twins’ Early Development Study, Robert Plomin. Grainne McLoughlin was supported by a fellowship from the National Institute for Health Research. Rebecca Pinto was supported by a Medical Research Council studentship. Isabelle Ouellet-Morin is a Canadian Institutes of Health Research new investigator award recipient.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, R., Rijsdijk, F., Ouellet-Morin, I. et al. The aetiological association between the dynamics of cortisol productivity and ADHD. J Neural Transm 123, 991–1000 (2016). https://doi.org/10.1007/s00702-016-1534-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1534-5