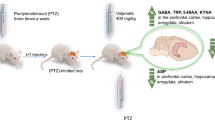
Abstract
Our study demonstrated that the development of seizures during the electrically induced kindling of seizures is associated with significant changes in the concentration of kynurenic acid (KYNA) and its precursor, tryptophan (TRP). The primary finding of our study was an increase in KYNA levels and the KYNA/TRP ratio (a theoretical index of activity of the kynurenine pathway) in the amygdala and hippocampus of kindled animals. We also found decreases in the concentration of tryptophan in the hippocampus and prefrontal cortex. Changes in the concentration of KYNA and TRP in the amygdala were accompanied by a significant decrease in γ-Aminobutryic Acid (GABA) levels and an increase in the glutamate/GABA ratio. Moreover, we found a significant negative correlation between the local concentrations of KYNA and glutamate in the amygdala of kindled rats. However, there were no changes in the local concentrations of the following amino acids: glutamate, aspartate, glutamine, glycine, taurine and alanine. In conclusion, these new results suggest a modulatory influence of KYNA on the process of epileptogenesis, characterized by a negative relationship between the KYNA and glutamate systems in the amygdala.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kindling of seizures, an animal model of temporal lobe epilepsy, was used to study the effects of repeated electrical stimulation on brain function. Repeated subthreshold brain stimulations gradually reduce the seizure threshold, culminating in tonic–clonic seizures. Electric stimulation is usually applied to certain brain areas, such as the amygdala, the hippocampus, the perforant path or the piriform cortex. Kindling can also be induced by the repeated administration of subthreshold doses of chemoconvulsants, such as pentylenetetrazol (PTZ) or picrotoxin.
Excessive activation of the excitatory pathways in the brain, including glutamate transmission and an imbalance between neuronal excitation and inhibition, is believed to be the contributing mechanism involved in epileptogenesis. It is well known that overactivated glutamate receptors participate in both the initiation and spread of epileptiform-like activity after kindling (McNamara et al. 1988). Moreover, N-methyl-d-aspartate (NMDA) receptor antagonists are effective anticonvulsants (Wong et al. 1986). Thus, it is commonly believed that overstimulated glutamatergic transmission, mediated primarily through the NMDA and other glutamatergic ionotropic receptors, significantly contributes to the development of the kindling phenomenon (McNamara et al. 1988).
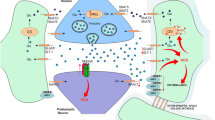
Similar to GABA and glycine, kynurenic acid (KYNA) is thought to be one of the most important endogenous inhibitory neuroactive metabolites controlling the kindling process and seizures (Turski et al. 1988, 1989, 1990). KYNA is synthesized from tryptophan by astrocytes and neurons through the transamination of its direct precursor l-kynurenine by four kynurenine aminotransferases in the kynurenine pathway (KAT I–IV) (Kiss et al. 2003).
KYNA is an endogenous antagonist of glutamate NMDA receptors (Erhardt et al. 2009). It also acts as a competitive antagonist at AMPA and kainate receptors and non-competitively blocks presynaptic α7 nicotinic acetylcholine receptors (α7 nAChRs), regulating glutamate release (Fu et al. 2000; Harris et al. 1998; Hilmas et al. 2001). A growing body of evidence suggests that KYNA is an important endogenous neuroprotective agent and that it prevents neuronal loss following various neuronal injuries, including excitotoxic or ischemia-induced damage (Cozzi et al. 1999; Sas et al. 2007). Recently, Rozsa et al. (2008) demonstrated that the application of l-kynurenine led to the inhibition of PTZ-induced discharges in hippocampal slices in vitro (Rozsa et al. 2008).
In addition, there are data indicating that the concentration of KYNA in the brain is altered in epilepsy. However, the nature and time response of changes in KYNA concentration and the relation of KYNA levels to the concentrations of other amino acids, including its precursor, tryptophan, are not elucidated (Heyes et al. 1994). It is particularly important to determine the possible role of an interaction between amino acids in cortical and limbic brain structures. The pivotal role of the hippocampus, amygdala and prefrontal cortex in epilepsy prompted the examination of changes in the concentration of KYNA and amino acids, including tryptophan, in these brain structures in rats subjected to electrically induced hippocampal kindling.
Methods
Animals
Twenty 12-week-old adult male Wistar rats weighing 220 ± 20 g were used in the study. Two rats were housed per cage under standard laboratory conditions with a 12-h photocycle (lights on at 7:00 a.m.), controlled temperature (20 ± 2°C) and humidity (70%) and free access to food and water. Experiments were performed between 9:00 a.m. and 3:00 p.m. The study was conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and was approved by the Committee for Animal Care and Use at the Medical University in Warsaw (Protocol No. 17/2006).
Surgical implantation of electrodes
After acclimatization to the laboratory conditions for a period of one week, the rats were anaesthetized with an intraperitoneal (i.p.) injection of ketamine 75 mg/kg (Ketanest, Parke Davis), and sodium pentobarbital 20 mg/kg (Morbital, Biovet).
One tri-polar electrode (MS333/1; Plastics One, USA) was implanted into the dorsal hippocampus of each rat using standard stereotaxic techniques. Two twisted stainless-steel wires implanted into the hippocampus served as stimulation/recording electrodes. The distance between the electrode tips was approximately 500 μm. One stainless steel wire, connected to a metal screw located over the parietal cortex, was used as a neutral electrode. All electrodes were implanted into the left or right dorsal hippocampus. The stereotaxic coordinates of the hippocampal implantation were (from the skull surface): bregma (−)2.4 mm, lateral 2.0 mm and ventral 3.5 mm (Fig. 1a; Paxinos and Watson 1998). The electrodes were held in place with dental acrylic cement applied to the exposed skull surface.
a Histological micrograph showing electrode placement. An arrow indicates the electrode tract. The slice was photographed with a ×4 objective lens and then magnified digitally. b Representative EEG recording from the hippocampus of an animal with stage 5 seizure after hippocampal electrical stimulations (1-s train of 60 Hz 1-ms monophasic square-wave pulses at an intensity of 500 μA)
Kindling and electroencephalographic (EEG) recording
Electrical stimulation of the hippocampus was initiated 2 weeks after surgery. The kindling stimuli were delivered daily for 5 days a week. Stimulations were performed by a Grass Model S88 stimulator connected to a constant-current unit (CCU1) and a stimulus-isolated unit (SIU5 RF; Grass Instruments). The kindling stimulus consisted of a 1-s train of 60 Hz and 1-ms monophasic square-wave pulses at an intensity of 500 μA until at least two consecutive fully kindled stage 5 seizures were elicited. The seizures were classified according to the Racine scale as follows: Stage 1, jaw clonus; Stage 2, head nodding; Stage 3, forelimb clonus; Stage 4, rearing on the hind limbs; and Stage 5, loss of postural control, tonic-clonic seizures (Racine et al. 1972). The number of stimulations necessary to achieve stage 5 seizures ranged between 45 and 60. Following each stimulation, the seizure stage and presence of afterdischarges (ADs) were recorded. A stimulation/recording switching unit (Grass Instruments) with the ability to choose the mode of operation (record or stimulation) was used. A Grass PolyVIEW16 data acquisition and analysis system (Grass Instruments) was used to record electroencephalographic (EEG) activity. Before the signal was recorded, it was amplified by the high performance amplifier 15A54 (Grass Instruments). An AD was defined as “spike” activity in the EEG (Fig. 1b), with an amplitude of at least twice the baseline activity and a duration of at least 5 s. One-and-a-half hours after the final stimulation, the kindled and control animals (sham stimulated rats, which had been implanted with electrodes and subjected to all experimental details) were killed, and their brains were removed, frozen at −70°C and cut into slices (1 mm thick). The prefrontal cortex [bregma (+)0.7], amygdala and hippocampus [bregma (−)3.6] were removed from the brain slices (within their defined anatomical limits) using a scalpel and then placed onto ice-cold Petri dishes aided by a magnifying glass. The amino acid composition and concentration of KYNA in these anatomical structures were subsequently analyzed.
Determination of kynurenic acid concentration in brain homogenates
To determine kynurenic acid (KYNA) and tryptophan (TRP) concentrations, each tissue sample was weighed, placed in a cool, dry polypropylene vial, and homogenized in 20 volumes of ice-cold 2% perchloric acid (30 s at 4°C). The homogenates were then centrifuged at 26,880×g for 8 min at 4°C. After centrifugation, the supernatants were collected and filtered through 0.45 μm filter (Millipore). Samples were immediately frozen and kept at −70°C until they were assayed. Kynurenic acid and tryptophan brain concentrations were measured according to the modified methods of Wu et al. (1992) and Herve et al. (1996). The detection of KYNA and tryptophan was performed by high-performance liquid chromatography (HPLC) with fluorescence detection. The HPLC system used for the analysis consisted of the following components: a pump (Shimadzu, LC-10AD VP) and a fluorescence detector (Shimadzu, RF-10 XL). The fluorescence detector was set at an excitation wavelength of 344 nm and emission wavelength of 398 nm for the detection of KYNA, and 404 and 504 nm for the detection of TRP. Supernatant samples were injected manually via a Rheodyne 7725i injection valve with a 20 μl sample loop. KYNA and TRP were separated on a Phenomenex Luna C18 (150 mm × 3 mm) column with a Phenomenex KJO-4286 precolumn set at a flow rate of 0.4 ml/min operating at room temperature. The mobile phase (isocratic system) consisted of 50 mM sodium acetate, 250 mM zinc acetate and 4% acetonitrile (adjusted with acetic acid to pH 6.2) and was degassed for 15 min. Chromatogram registration and analysis were performed using ChromaX 2004 software. The retention time of KYNA and tryptophan under these conditions was 9 and 17 min, respectively. Calibration curves were created by injecting KYNA and TRP standards in concentrations ranging from 0.5 to 120 nM and 0.5–20 μM, respectively. The concentrations of KYNA and TRP were calculated as pmol/g of tissue or nmol/g of tissue respectively.
Determination of amino acids in homogenates
For determination of amino acids, each tissue sample was weighed, placed in a cool, dry polypropylene vial, and homogenized in 20 volumes of ice-cold 2% perchloric acid for 30 s at 4°C. The homogenates were then centrifuged at 26,880×g for 8 min at 4°C. After centrifugation, the supernatants were collected and filtered through a 0.45 μm filter (Millipore). Samples were immediately frozen and kept at −70°C until assayed. The concentrations of amino acids (glutamate, GABA, alanine, glutamine, glycine, aspartate, taurine and the glutamate to GABA ratio) were determined by high performance liquid chromatography (HPLC), with electrochemical detection (Shimadzu) after a derivatization procedure, as described previously (Szyndler et al. 2008). Shortly thereafter, compounds were eluted isocratically with the mobile phase delivered at 0.70 ml/min using a Shimadzu Class VP LC 10 AD pump. A Rheodyne injection valve with a 20 μl sample loop was used to manually inject the samples. The preparation of the mobile phase and the derivatizing agents were based on a method by Rowley et al. (1995), with modifications. The mobile phase consisted of 45 mM disodium phosphate and 0.15 mM EDTA with 25% (v/v) methanol adjusted to pH 3.9 using 0.2 M citric acid. To obtain agents for derivatization, OPA (2 mg, Fluka, Germany) was dissolved in 0.5 ml 1 M Na2SO3, 0.5 ml methanol, and 0.9 ml sodium tetraborate buffer (0.1 M) adjusted to pH 10.4 with 5 M sodium hydroxide. The concentrations of amino acids were calculated as μmol/g of brain tissue.
Statistics
The statistical package, Statistica for Windows Release 8 (StatSoft Inc., USA), was used for statistical analysis. The differences in amino acid and KYNA concentrations among the experimental groups (kindled vs. controls) were analyzed with the Student’s t test. The correlation between the concentration of amino acids and KYNA in the same animals was performed using Pearson’s r correlation test; a cut-off of P < 0.05 was used to indicate statistical significance.
Results
Student’s t test revealed that in the amygdala the fully kindled rats showed a significantly decreased concentration of GABA (t = 2.55, P = 0.024) and an increased GLU/GABA ratio (t = 3.26, P = 0.006) (Fig. 2a). The concentration of KYNA (t = 4.03, P = 0.001) and the KYNA/TRP ratio (t = 3.88, P = 0.001) were increased compared to the control group (Fig. 2b). In the hippocampus, an increased concentration of KYNA (t = 2.41, P = 0.027) and KYNA/TRP ratio (t = 2.69, P = 0.016), as well as a decreased concentration of tryptophan (t = 2.32, P = 0.033), were found (Fig. 3b). In the prefrontal cortex of the fully kindled rats, only a decrease in the concentration of tryptophan (t = 3.24, P = 0.005, Fig. 4b) was apparent. No changes in the local concentrations of other amino acids (aspartate, glutamine, glycine, taurine and alanine) were present (Figs. 2a, 3a, 4a). In the current study, we found a significant negative correlation between the local concentrations of KYNA and glutamate in the amygdala of kindled rats (r = −0.7; P < 0.05). No other significant correlations between KYNA and amino acid concentrations were observed.
a Concentration of taurine (TAU), glutamine (GLM), glycine (GLY), aspartate (ASP), glutamate (GLU), alanine (ALA) and GABA and the GLU/GABA ratio in the amygdala. b Concentration of kynurenic acid (KYNA) and tryptophan (TRP) and the KYNA/TRP ratio in the kindled rats. The ratio (KYNA/TRP) was multiplied by 1,000 to adjust the result to the log scale of the figure. The number of rats in the experimental groups varied from 8 to 10. Data represent the mean ± SEM. *Differs from the control group. *P < 0.05; **P < 0.01; ***P < 0.005
a Concentration of taurine (TAU), glutamine (GLM), glycine (GLY), aspartate (ASP), glutamate (GLU), alanine (ALA) and GABA and the GLU/GABA ratio in the hippocampus. b Concentration of kynurenic acid (KYNA) and tryptophan (TRP) and the KYNA/TRP ratio in the kindled rats. The ratio (KYNA/TRP) was multiplied by 1,000 to adjust the result to the log scale of the figure. The number of rats in the experimental groups varied from 8 to 10. Data represent the mean ± SEM. *Differs from control group. *P < 0.05
a Concentrations of taurine (TAU), glutamine (GLM), glycine (GLY), aspartate (ASP), glutamate (GLU), alanine (ALA), and GABA and the GLU/GABA ratio in the prefrontal cortex. b Concentration of kynurenic acid (KYNA) and tryptophan (TRP) and the KYNA/TRP ratio in the kindled rats. The ratio (KYNA/TRP) was multiplied by 1,000 to adjust the result to the log scale of the figure. The number of rats in the experimental groups varied from 8 to 10. Data represent the mean ± SEM. *Differs from control group. ***P < 0.005
Discussion
Our study demonstrated that the development of seizures during the electrically induced hippocampal kindling of seizures is associated with significant changes in the concentration of KYNA and its precursor, tryptophan. Thus, the new finding of our study indicates that an increase in KYNA levels and the KYNA/TRP ratio (a theoretical index of activity of kynurenine pathway) occurred in the amygdala and hippocampus of kindled animals. We also found that the tryptophan concentration in the hippocampus and prefrontal cortex decreased. Changes in the amygdala concentration of KYNA and TRP were accompanied by a significant decrease in GABA levels and an increase in the GLU/GABA ratio. A significant negative correlation between the concentrations of KYNA and glutamate in the amygdala of kindled rats was found. There were no changes in the concentrations of the following amino acids: glutamate, aspartate, glycine, glutamine, taurine and alanine.
Our study indicated that the process of kindling increases the activity of the kynurenine pathway of tryptophan metabolism. This may account for the increase in the KYNA concentration and the KYNA/TRP ratio as well as the decrease in tryptophan levels. In a previous study, KYNA levels in electrically kindled animals were also found to be increased in the hippocampus (Wu et al. 1995). The increase in KYNA levels in the extracellular fluid (ECF) was not dependent on electrical stimulation per se but on the process of kindling development and neuronal and/or astrocytic reorganization (Wu et al. 1995). In contrast, Löscher et al. (1996) demonstrated that there were no significant changes in brain tissue KYNA concentrations, with the exception of the nucleus accumbens, where kindling induced a lasting increase in kynurenate. Recently, we reported a significant decrease in tissue KYNA concentrations in some brain structures (the caudate putamen, entorhinal cortex, piriform cortex, amygdala and hippocampus) after PTZ-induced kindling (Maciejak et al. 2009).
Previously, using several models of epilepsy, it was demonstrated that chemoconvulsant-induced seizures (including PTZ) are associated with an immediately occurring release and long-lasting elevation of extracellular KYNA levels (Wu and Schwarcz 1996). Given that PTZ has a very short half-life, the reported biochemical effect was likely not due to its direct influence on the KYNA metabolism but was the result of PTZ-induced seizures. Of note, the electrical stimulation by depolarization stimulates, in a nonspecific manner, all neuronal systems, and astrocytes in the epileptic focus. The initial mechanism of PTZ action involves only a single GABAergic neurotransmitter system. Hence, it is possible that the repeated blockade of GABA-A receptors located on neurons and astrocytes could lead to the other adaptive biochemical effects in comparison with electrical brain stimulation. Furthermore, it is conceivable that, while acute seizures should increase the ECF concentration of KYNA, repeated brain stimulation could lead to the depletion of KYNA tissue stores. The present findings indicate, however, that, contrary to this assumption, chronic electrical stimulation of the hippocampus led to the increase in tissue KYNA concentration. These findings suggest that changes in KYNA levels may depend on the model of temporal lobe epilepsy (chemically or electrically induced) and the time of biochemical analysis in relation to the seizure episode.
Brain KYNA concentration may be regulated by several of the following mechanisms: the uptake of tryptophan and kynurenine (precursors of KYNA); the activity of indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO), which are enzymes responsible for the rate-limiting degradation of tryptophan to kynurenine; the efficiency of the transamination of kynurenine to KYNA by KAT; and the liberation of KYNA from the synthesis origin (Wu et al. 1995). The exact mechanism of the alterations in brain KYNA levels during electrical kindling was not fully elucidated. It has been suggested that the lasting elevation of KYNA levels in fully kindled animals appears to be different from the increase that occurs immediately following generalized convulsions and may include a desensitization of presynaptic glial amino acid receptors, regulating local KYNA release, in response to repeatedly occurring glutamate release (Wu and Schwarcz 1996).
There is strong evidence that KYNA affects the development of kindled seizures. Thompson et al. (1988) demonstrated that intracerebroventricular administration of KYNA (360 nmol) prior to the administration of the electrical kindling stimulus significantly reduced the rate of kindling (Thompson et al. 1988). Similarly, a significant increase in KYNA levels in the brain, evoked by the administration of kynurenine hydroxylase and kynureninase inhibitors, protected rats from maximal electroshock-induced seizures or from audiogenic seizures in DBA/2 mice (Carpenedo et al. 1994). An increase in KYNA concentration was observed one week after status epilepticus as well as in the extracellular fluid in the fully kindled animals in vivo 1 week after the last stimulation (Du et al. 1993; Wu et al. 1995). Simultaneously, there were no changes in the KYNA levels in the animals with stage 2 kindled seizures. Considering the afterdischarges duration did not differ in animals with stage 2 and in the fully kindled animals, it appeared that the increase in KYNA in the fully kindled animals was not simply a consequence of electrical activity. Mechanisms responsible for this KYNA increase were not clear (Wu et al. 1995). Some studies indicated astrocytic hypertrophy in brain structures (e.g. in the hippocampal formation) during the kindling of seizures and, as a result, an increase in the activity of KAT, the primary enzyme responsible for KYNA synthesis (Wu et al. 1995). Thus, the process of kindling and astrocytic changes was accompanied by an increase in KYNA (Du et al. 1993). Hypertrophy of astrocytes is likely elicited by neuronal injury because highly hypertrophied astrocytes were found in brain areas where neurodegeneration occurred. However, it should be noted that Wu et al. (1995) reported no significant changes in KAT activity in the amygdala of animals that were electrically kindled (Wu et al. 1995).
In our work, we sought to determine whether changes in KYNA levels were associated with changes in tryptophan metabolism. Biochemical analysis demonstrated that an increase in KYNA levels in the hippocampus was accompanied by a decrease in tryptophan concentration and an increase in the KYNA/TRP ratio. These data suggested that the kynurenine pathway was significantly activated during kindling; however, the exact mechanism responsible for this phenomenon is difficult to pinpoint. The increase in KYNA could be related to many of the aforementioned factors occurring in parallel (changes in synthesis, degradation, or release). Further studies on changes in the activity of the kynurenine pathway are warranted to clarify the intrinsic mechanisms of this phenomenon. This becomes particularly important in light of our recent data (unpublished), which demonstrated that an important and widely used antiepileptic drug, valproic acid, given at a dose of 400 mg/kg to rats fully inhibited PTZ-induced seizures (50 mg/kg, i.p.) and caused more than a tenfold increase in the ECF concentration of KYNA (in vivo). Furthermore, there is evidence suggesting that an increase in KYNA concentration, evoked by the administration of kynurenine hydroxylase inhibitors, exerts an important neuroprotective action that prevents neuronal loss following ischemia (Cozzi et al. 1999). The neuroprotective potency of KYNA may be due to its antagonist actions at glutamate receptors or the blockade of glutamate release by the inhibition of presynaptic α7 nAChRs in glutamatergic terminals (Fu et al. 2000; Hilmas et al. 2001; Harris et al. 1998; Cozzi et al. 1999).
In our study, we also found a decrease in GABA levels and an increase in the glutamate/GABA ratio in the amygdala. This finding suggested that changes in local KYNA levels may be an adaptive reaction to the disrupted balance between the primary inhibitory and excitatory brain systems. The function of the GABA system can be substantially modified by seizures, and both the stimulation and the inhibition of local GABAergic activity in response to kindling has been reported (Kamphuis et al. 1990; Kaura et al. 1995). Recently, we found that PTZ-induced kindling reduced the extracellular levels of GABA (in vivo) in the rat hippocampus (Szyndler et al. 2008). The data presented here confirmed the previous findings of our group and others, which indicated that the imbalance between glutamatergic, GABAergic, and KYNA synaptic transmission characterizes hyperexcitable, epileptic and local neuronal circuits. According to this suggestion, we found a significant negative correlation between the local concentration of KYNA and glutamate in the amygdala of kindled rats. More experiments are needed to elucidate the problem of a contribution of KYNA to the process of epileptogenesis. For example, it would be of interest to repeat the kindling procedure in rats given a low tryptophan diet. Would the lack of an increase in KYNA specifically affect kindling and would it simultaneously compensate for the increase in GABA concentration?
Also worth mentioning is the fact that tryptophan is the precursor not only of KYNA but of several other neuroactive substances like serotonin and melatonin. Moreover, the effects of 5-HT on neuronal excitability depend on which 5-HT receptors are activated. In this context, one ought to notice earlier studies performed with the help of in situ microvoltametry and neuromolecular imaging using the resected tissue samples obtained from patients with mesial (MTLE) and neocortical temporal lobe epilepsy (NTLE). The authors found in granular cells and pyramidal layer of dentate gyrus, in contrast to our study, that l-tryptophan concentrations were significantly higher in MTLE patients in comparison with NTLE patients (Pacia and Broderick 2005), while 5-HT concentrations were higher in NTLE versus MTLE (Broderick et al. 2000). Although, these clinical data cannot be directly compared with the current results, all these findings indicate that l-tryptophan metabolism may lead to the formation of various centrally active substances with pro- and anticonvulsant properties, thus contributing to the pathogenesis of different types of epilepsy.
In conclusion, the results presented here provide support for the modulatory role of KYNA during the kindling of seizures in limbic structures. These new results suggest an activation of the kynurenine pathway in the brain. The potential participation of KYNA in the process of epileptogenesis was demonstrated by a negative relationship between KYNA and glutamate levels in the amygdala.
References
Broderick PA, Pacia SV, Doyle WK, Devinsky O (2000) Monoamine neurotransmitters in resected hippocampal subparcellations from neocortical and mesial temporal lobe epilepsy patients: in situ microvoltammetric studies. Brain Res 878:48–63
Carpenedo R, Chiarugi A, Russi P, Lombardi G, Carlà V, Pellicciari R, Mattoli L, Moroni F (1994) Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience 61:237–243
Cozzi A, Carpenedo R, Moroni F (1999) Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3, 4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61–8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab 19:771–777
Du F, Schmidt W, Okuno E, Kido R, Köhler C, Schwarcz R (1992) Localization of kynurenine aminotransferase immunoreactivity in the rat hippocampus. J Comp Neurol 321:477–487
Du F, Williamson J, Bertram E, Lothman E, Okuno E, Schwarcz R (1993) Kynurenine pathway enzymes in a rat model of chronic epilepsy: immunohistochemical study of activated glial cells. Neuroscience 55:975–989
Erhardt S, Olsson SK, Engberg G (2009) Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs 23:91–101
Fu Y, Matta SG, Gao W, Sharp BM (2000) Local alpha-bungarotoxin-sensitive nicotinic receptors in the nucleus accumbens modulate nicotine-stimulated dopamine secretion in vivo. Neuroscience 101:369–375
Harris CA, Miranda AF, Tanguay JJ, Boegman RJ, Beninger RJ, Jhamandas K (1998) Modulation of striatal quinolinate neurotoxicity by elevation of endogenous brain kynurenic acid. Br J Pharmacol 124:391–399
Herve C, Beyne P, Jamault H, Delacoux E (1996) Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J Chromatogr B Biomed Appl 675:157–161
Heyes MP, Saito K, Devinsky O, Nadi NS (1994) Kynurenine pathway metabolites in cerebrospinal fluid and serum in complex partial seizures. Epilepsia 35:251–257
Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001) The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 21:7463–7473
Kamphuis W, Huisman E, Dreijer AM, Ghijsen WE, Verhage M, Lopes da Silva FH (1990) Kindling increases the K(+)-evoked Ca2(+)-dependent release of endogenous GABA in area CA1 of rat hippocampus. Brain Res 511:63–70
Kaura S, Bradford HF, Young AMJ, Croucher MJ, Hughes PD (1995) Effect of amygdaloid kindling on the content and release of amino acids from the amygdaloid complex: in vivo and in vitro studies. J Neurochem 65:1240–1249
Kiss C, Ceresoli-Borroni G, Guidetti P, Zielke CL, Zielke HR, Schwarcz R (2003) Kynurenate production by cultured human astrocytes. J Neural Transm 110:1–14
Löscher W, Ebert U, Lehmann H (1996) Kindling induces a lasting, regionally selective increase of kynurenic acid in the nucleus accumbens. Brain Res 725:252–256
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Taracha E, Skórzewska A, Lehner M, Bidziński A, Płaźnik A (2009) Time course of changes in the concentration of kynurenic acid in the brain of pentylenetetrazol-kindled rats. Brain Res Bull 78:299–305
McNamara JO, Russell RD, Rigsbee L, Bonhaus DW (1988) Anticonvulsant and antiepileptogenic actions of MK-801 in the kindling and electroshock models. Neuropharmacology 27:563–568
Pacia SV, Broderick PA (2005) Bioimaging l-tryptophan in human hippocampus and neocortex. In: Broderick PA, Rahni DN, Kolodny EH (eds) Bioimaging in neurodegeneration. Humana Press, Springer Press, NJ, pp 141–147
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Racine RJ, Gartner JG, Burnham WM (1972) Epileptiform activity and neural plasticity in limbic structures. Brain Res 47:262–268
Rowley HL, Martin KF, Marsden CA (1995) Determination of in vivo amino acids neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods 5:93–99
Rozsa E, Robotka H, Nagy D, Farkas T, Sas K, Vecsei L, Toldi J (2008) The pentylenetetrazole-induced activity in the hippocampus can be inhibited by the conversion of l-kynurenine to kynurenic acid: an in vitro study. Brain Res Bull 76:474–479
Sas K, Robotka H, Toldi J, Vécsei L (2007) Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci 257:221–239
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Lehner M, Taracha E, Walkowiak J, Skórzewska A, Wisłowska-Stanek A, Hamed A, Bidziński A, Płaźnik A (2008) Changes in the concentration of amino acids in the hippocampus of pentylenetetrazole-kindled rats. Neurosci Lett 439:245–249
Thompson JL, Holmes GL, Taylor GW, Feldman DR (1988) Effects of kynurenic acid on amygdaloid kindling in the rat. Epilepsy Res 2:302–308
Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO Jr, Schwarcz R (1988) Identification and quantification of kynurenic acid in human brain tissue. Brain Res 454:164–169
Turski WA, Gramsbergen JB, Traitler H, Schwarcz R (1989) Rat brain slices produce and liberate kynurenic acid upon exposure to l-kynurenine. J Neurochem 52:1629–1636
Turski WA, Urbanska E, Dziki M, Parada-Turska J, Ikonomidou C (1990) Excitatory amino acid antagonists protect mice against seizures induced by bicuculline. Brain Res 514:131–134
Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL (1986) The anticonvulsant MK-801 is a potent N-methyl-d-aspartate antagonist. Proc Natl Acad Sci USA 83:7104–7108
Wu HQ, Schwarcz R (1996) Seizure activity causes elevation of endogenous extracellular kynurenic acid in the rat brain. Brain Res Bull 39:155–162
Wu HQ, Baran H, Ungerstedt U, Schwarcz R (1992) Kynurenic acid in the quinolinate-lesioned rat hippocampus: studies in vitro and in vivo. Eur J Neurosci 4:1264–1270
Wu HQ, Monno A, Schwarcz R, Vezzani A (1995) Electrical kindling is associated with a lasting increase in the extracellular levels of kynurenic acid in the rat hippocampus. Neurosci Lett 198:91–94
Acknowledgments
This study was supported by grant No. N40102632/0640 from the Ministry of Science and Higher Education, Warsaw, Poland and by a statutory grant No. 501-003-11043 from the Institute of Psychiatry and Neurology, Warsaw, Poland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Szyndler, J., Maciejak, P., Turzyńska, D. et al. The effects of electrical hippocampal kindling of seizures on amino acids and kynurenic acid concentrations in brain structures. J Neural Transm 119, 141–149 (2012). https://doi.org/10.1007/s00702-011-0700-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-011-0700-z