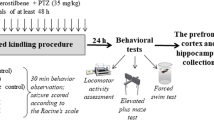
Abstract
Background
The study aimed to assess the influence of a single valproate (VPA) administration on inhibitory and excitatory neurotransmitter concentrations in the brain structures involved in epileptogenesis in pentylenetetrazol (PTZ)-kindled rats.
Methods
Adult, male Wistar rats were kindled by repeated intraperitoneal (ip) injections of PTZ at a subconvulsive dose (30 mg/kg, three times a week). Due to the different times required to kindle the rats (18–22 injections of PTZ), a booster dose of PTZ was administrated 7 days after the last rats were kindled. Then rats were divided into two groups: acute administration of VPA (400 mg/kg) or saline given ip. The concentration of amino acids, kynurenic acid (KYNA), monoamines, and their metabolites in the prefrontal cortex, hippocampus, amygdala, and striatum was assessed by high-pressure liquid chromatography (HPLC).
Results
It was found that a single administration of VPA increased the gamma-aminobutyric acid (GABA), tryptophan (TRP), 5-hydroxyindoleacetic acid (5-HIAA), and KYNA concentrations and decreased aspartate (ASP) levels in PTZ-kindled rats in the prefrontal cortex, hippocampus, amygdala and striatum.
Conclusions
Our results indicate that a single administration of VPA in the PTZ-kindled rats restored proper balance between excitatory (decreasing the level of ASP) and inhibitory neurotransmission (increased concentration GABA, KYNA) and affecting serotoninergic neurotransmission in the prefrontal cortex, hippocampus, amygdala, and striatum.
Graphical abstract




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- 3-MT:
-
3-Methoxytyramine
- 5-HIAA:
-
5-Hydroxyindoleacetic acid
- 5-HT:
-
Serotonin
- ALA:
-
Alanine
- ASP:
-
Aspartate
- DA:
-
Dopamine
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- GABA:
-
Gamma-aminobutyric acid
- GABA-A:
-
Gamma-aminobutyric acid type A
- GLU:
-
Glutamate
- GLN:
-
Glutamine
- GLY:
-
Glycine
- HPLC:
-
High-pressure liquid chromatography
- HVA:
-
Homovanillic acid
- ip :
-
Intraperitoneal
- KYNA:
-
Kynurenic acid
- NA:
-
Noradrenaline
- NMDA:
-
N-Methyl-D-aspartate
- PTZ:
-
Pentylenetetrazol
- SEM:
-
Standard error of the mean
- TAU:
-
Taurine
- TRP:
-
Tryptophan
- VPA:
-
Valproate
References
Geraghty JR, Senador D, Maharathi B, Butler MP, Sudhakar D, Smith RA, et al. Modulation of locomotor behaviors by location-specific epileptic spiking and seizures. Epilepsy Behav. 2021;114(Pt A): 107652. https://doi.org/10.1016/j.yebeh.2020.107652.
Pack AM. Epilepsy overview and revised classification of seizures and epilepsies. Continuum (Minneap Minn). 2019;25(2):306–21. https://doi.org/10.1212/CON.0000000000000707.
Engelborghs S, D’Hooge R, Deyn PPD. Pathophysiology of epilepsy. Acta Neurol Belg. 2000;100(4):201–13.
Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci. 2012. https://doi.org/10.1002/0471142301.ns0937s58.
White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38(Suppl 1):S9-17. https://doi.org/10.1111/j.1528-1157.1997.tb04523.x.
Yuskaitis CJ, Rossitto LA, Groff KJ, Dhamne SC, Zhang B, Lalani LK, et al. Factors influencing the acute pentylenetetrazole-induced seizure paradigm and a literature review. Ann Clin Transl Neurol. 2021;8(7):1388–97. https://doi.org/10.1002/acn3.51375.
Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60.
Erum JV, Dam DV, Deyn PPD. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav. 2019;95:51–5.
Singh T, Mishra A, Goel RK. PTZ kindling model for epileptogenesis, refractory epilepsy, and associated comorbidities: relevance and reliability. Metab Brain Dis. 2021;36(7):1573–90. https://doi.org/10.1007/s11011-021-00823-3.
Ahmadi M, Dufour JP, Seifritz E, Mirnajafi-Zadeh J, Saab BJ. The PTZ kindling mouse model of epilepsy exhibits exploratory drive deficits and aberrant activity amongst VTA dopamine neurons in both familiar and novel space. Behav Brain Res. 2017;14(330):1–7. https://doi.org/10.1016/j.bbr.2017.05.025. (Epub 2017 May 12 PMID: 28506618).
Davis R, Peters DH, McTavish D. Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1994;47(2):332–72 (PMID: 7512905).
Kessler SK, McGinnis E. A practical guide to treatment of childhood absence epilepsy. Paediatr Drugs. 2019;21(1):15–24. https://doi.org/10.1007/s40272-019-00325-x.
Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016;15(2):210–8. https://doi.org/10.1016/S1474-4422(15)00314-2.
Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, Calabresi P, Costa C. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019;17(10):926–46. https://doi.org/10.2174/1570159X17666181227165722.
Litvinova SA, Voronina TA, Kondrakhin EA, Gaydukov IO, Davletshin AI, Vasileva EV, et al. ERK1/2 kinases and dopamine D2 receptors participate in the anticonvulsant effects of a new derivative of benzoylpyridine oxime and valproic acid. Eur J Pharmacol. 2021;903: 174150. https://doi.org/10.1016/j.ejphar.2021.174150. (Epub 2021 May 5 PMID: 33961874).
Miziak B, Konarzewska A, Ułamek-Kozioł M, Dudra-Jastrzębska M, Pluta R, Czuczwar SJ. Anti-epileptogenic effects of antiepileptic drugs. Int J Mol Sci. 2020;21(7):2340. https://doi.org/10.3390/ijms21072340.
Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic administration of valproic acid reduces brain NMDA signaling via arachidonic acid in unanesthetized rats. Neurochem Res. 2008;33(11):2229–40. https://doi.org/10.1007/s11064-008-9700-2.
Davies JA. Mechanisms of action of antiepileptic drugs. Seizure. 1995;4:267–71. https://doi.org/10.1016/s1059-1311(95)80003-4.
Nau H, Löscher W. Valproic acid: brain and plasma levels of the drug and its metabolites, anticonvulsant effects and gamma-aminobutyric acid (GABA) metabolism in the mouse. J Pharmacol Exp Ther. 1982;220(3):654–9.
Tunnicliff G. Actions of sodium valproate on the central nervous system. J Physiol Pharmacol. 1999;50(3):347–65.
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Płaźnik A. Kynurenic acid: a new effector of valproate action? Pharmacol Rep. 2011;63(6):1569–73. https://doi.org/10.1016/s1734-1140(11)70723-x.
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Kołosowska K, Lehner M, et al. The kynurenine pathway: a missing piece in the puzzle of valproate action? Neuroscience. 2013;234:135–45. https://doi.org/10.1016/j.neuroscience.2012.12.052.
Maciejak P, Szyndler J, Kołosowska K, Turzyńska D, Sobolewska A, Walkowiak J, et al. Valproate disturbs the balance between branched and aromatic amino acids in rats. Neurotox Res. 2014;25(4):358–68. https://doi.org/10.1007/s12640-013-9441-0.
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Bidziński A, Kołosowska K, et al. Time course of changes in the concentration of amino acid in the brain structures of pentyleneterazole-kindled rats. Brain Res. 2010;1342:150–9. https://doi.org/10.1016/j.brainres.2010.04.045.
Joshi D, Katyal J, Arava S, Gupta YK. Effects of enalapril and losartan alone and in combination with sodium valproate on seizures, memory, and cardiac changes in rats. Epilepsy Behav. 2019;92:345–52. https://doi.org/10.1016/j.yebeh.2018.12.019.
Kołosowska K, Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Płaźnik A. The role of interleukin-1β in the pentylenetetrazole-induced kindling of seizures, in the rat hippocampus. Eur J Pharmacol. 2014;731:31–7. https://doi.org/10.1016/j.ejphar.2014.03.008.
Töllner K, Twele F, Löscher W. Evaluation of the pentylenetetrazole seizure threshold test in epileptic mice as surrogate model for drug testing against pharmacoresistant seizures. Epilepsy Behav. 2016;57(Pt A):95–104. https://doi.org/10.1016/j.yebeh.2016.01.032.
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Bidziński A, Płaźnik A. Time course of changes in the concentrates of monoamines in the brain structures of pentylenetetrazole-kindled rats. J Neural Transm. 2010;117:707–18. https://doi.org/10.1007/s00702-010-0414-7.
Wlaź P, Ebert U, Potschka H, Löscher W. Electrical but not chemical kindling increases sensitivity to some phencyclidine-like behavioral effects induced by the competitive NMDA receptor antagonist D-CPPene in rats. Eur J Pharmacol. 1998;353(2–3):177–89. https://doi.org/10.1016/s0014-2999(98)00409-9.
Paxinos G, Watson CH. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998.
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Walkowiak J, Płaźnik A. The effects of electrical hippocampal kindling of seizures on amino acids and kynurenic acid concentrations in brain structures. J Neural Transm. 2012;119:141–9. https://doi.org/10.1007/s00702-011-0700-z.
Rowley HL, Martin KF, Marsden CA. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods. 1995;57(1):93–9. https://doi.org/10.1016/0165-0270(94)00132-z.
Wu HQ, Monno A, Schwarcz R, Vezzani A. Electrical kindling is associated with a lasting increase in the extracellular levels of kynurenic acid in the rat hippocampus. Neurosci Lett. 1995;198(2):91–4. https://doi.org/10.1016/0304-3940(95)11971-x.
Hervé C, Beyne P, Jamault H, Delacoux E. Determination of tryptophan and its kynurenine pathway metabolites in human serum by high-performance liquid chromatography with simultaneous ultraviolet and fluorimetric detection. J Chromatogr B Biomed Appl. 1996;675(1):157–61. https://doi.org/10.1016/0378-4347(95)00341-x.
Kaneda N, Asano M, Nagatsu T. Simple method for the simultaneous determination of acetylcholine, choline, noradrenaline, dopamine and serotonin in brain tissue by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1986;360(1):211–8. https://doi.org/10.1016/s0021-9673(00)91664-9.
Deng Y, Wang M, Wang W, Ma C, He N. Comparison and effects of acute lamotrigine treatment on extracellular excitatory amino acids in the hippocampus of PTZ-kindled epileptic and PTZ-induced status epilepticus rats. Neurochem Res. 2013;38(3):504–11. https://doi.org/10.1007/s11064-012-0942-7.
Flores-Soto M, Romero-Guerrero C, Vázquez-Hernández N, Tejeda-Martínez A, Martín-Amaya-Barajas FL, et al. Pentylenetetrazol-induced seizures in adult rats are associated with plastic, changes to the dendritic spines on hippocampal CA1 pyramidal neurons. Behav Brain Res. 2021;406: 113198. https://doi.org/10.1016/j.bbr.2021.113198.
Ciltas AC, Toy CE, Güneş H, Yaprak M. Effects of probiotics on GABA/glutamate and oxidative stress in PTZ- induced acute seizure model in rats. Epilepsy Res. 2023;195: 107190. https://doi.org/10.1016/j.eplepsyres.2023.107190.
Li ZP, Zhang XY, Lu X, Zhong MK, Ji YH. Dynamic release of amino acid transmitters induced by valproate in PTZ-kindled epileptic rat hippocampus. Neurochem Int. 2004;44(4):263–70. https://doi.org/10.1016/s0197-0186(03)00148-7.
Lasoń W, Chlebicka M, Rejdak K. Research advances in basic mechanisms of seizures and antiepileptic drug action. Pharmacol Rep. 2013;65(4):787–801. https://doi.org/10.1016/s1734-1140(13)71060-0.
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Taracha E, Skórzewska A, et al. Time course of changes in the concentration of kynurenic acid in the brain of pentylenetetrazol-kindled rats. Brain Res Bull. 2009;78(6):299–305. https://doi.org/10.1016/j.brainresbull.2008.10.010.
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Taracha E, Skórzewska A, et al. Mapping of c-Fos expression in the rat brain during the evolution of pentylenetetrazol-kindled seizures. Epilepsy Behav. 2009;16(2):216–24. https://doi.org/10.1016/j.yebeh.2009.07.030.
Nieoczym D, Socała K, Zelek-Molik A, Pieróg M, Przejczowska-Pomierny K, Szafarz M, et al. Anticonvulsant effect of pterostilbene and its influence on the anxiety- and depression-like behavior in the pentetrazol-kindled mice: behavioral, biochemical, and molecular studies. Psychopharmacology. 2021;238(11):3167–81. https://doi.org/10.1007/s00213-021-05933-5.
Sendrowski K, Sobaniec W. Hippocampus, hippocampal sclerosis and epilepsy. Pharmacol Rep. 2013;65(3):555–65. https://doi.org/10.1016/s1734-1140(13)71033-8.
Sierra A, Gröhn O, Pitkänen A. Imaging microstructural damage and plasticity in the hippocampus during epileptogenesis. Neuroscience. 2015;19(309):162–72. https://doi.org/10.1016/j.neuroscience.2015.04.054.
Engel J Jr. Epileptogenesis, traumatic brain injury, and biomarkers. Neurobiol Dis. 2019;123:3–7. https://doi.org/10.1016/j.nbd.2018.04.002.
Li L, He L, Harris N, Zhou Y, Engel J Jr, Bragin A. Topographical reorganization of brain functional connectivity during an early period of epileptogenesis. Epilepsia. 2021;62(5):1231–43. https://doi.org/10.1111/epi.16863.
Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78(2–3):102–16. https://doi.org/10.1016/j.eplepsyres.2007.11.011.
de Souza AG, Chaves Filho AJM, Souza Oliveira JV, de Souza DAA, Lopes IS, de Carvalho MAJ, et al. Prevention of pentylenetetrazole-induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: involvement of brain antioxidant and BDNF upregulating properties. Biomed Pharmacother. 2019;109:429–39. https://doi.org/10.1016/j.biopha.2018.10.066. (Epub 2018 Nov 3 PMID: 30399578).
Tokudome K, Okumura T, Shimizu S, Mashimo T, Takizawa A, Serikawa T, et al. Synaptic vesicle glycoprotein 2A (SV2A) regulates kindling epileptogenesis via GABAergic neurotransmission. Sci Rep. 2016;6:27420. https://doi.org/10.1038/srep27420.PMID:27265781;PMCID:PMC4893657.
Tchekalarova J, Sotiriou E, Angelatou F. Down-regulation of dopamine D1 and D2 receptors in the basal ganglia of PTZ kindling model of epilepsy: effects of angiotensin IV. Brain Res. 2004;1024(1–2):159–66. https://doi.org/10.1016/j.brainres.2004.07.060. (PMID: 15451378).
Yu Y, Xie W, Wang C. Chaihushugan decoction exerts antiepileptic effects by increasing hippocampal glutamate metabolism in pentylenetetrazole-kindled rats. J Tradit Chin Med. 2015;35(6):659–65. https://doi.org/10.1016/s0254-6272(15)30156-4.
Chapman AG, Hart GP. Anticonvulsant drug action and regional neurotransmitter amino acid changes. J Neural Transm. 1988;72(3):201–12. https://doi.org/10.1007/BF01243420.
Tamboli AM, Rub RA, Ghosh P, Bodhankar SL. Antiepileptic activity of lobeline isolated from the leaf of Lobelia nicotianaefolia and its effect on brain GABA level in mice. Asian Pac J Trop Biomed. 2012;2(7):537–42. https://doi.org/10.1016/S2221-1691(12)60092-6.
Nishikawa T, Yamamoto N, Tsuchida H, Umino A, Kawaguchi N. Endogenous D-serine in mammalian brains. Nihon Shinkei Seishin Yakurigaku Zasshi. 2000;20(1):33–9.
Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Lehner M, Taracha E, et al. Changes in the concentration of amino acids in the hippocampus of pentylenetetrazole-kindled rats. Neurosci Lett. 2008;439(3):245–9. https://doi.org/10.1016/j.neulet.2008.05.002.
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Kołosowska K, Krząścik P, et al. Is the interaction between fatty acids and tryptophan responsible for the efficacy of a ketogenic diet in epilepsy? The new hypothesis of action. Neuroscience. 2016;313:130–48. https://doi.org/10.1016/j.neuroscience.2015.11.029.
Jobe PC, Hem Ko KHL, Ray T, Dailey JW. Abnormalities in monoamine levels in the central nervous system in the genetically epilepsy-prone rats. Epilepsia. 1982;23:359–66. https://doi.org/10.1111/j.1528-1157.1982.tb05421.x.
Corcoran ME, Teskey GC. Chapter: models characteristic and mechanism of kindling. In: Schwartzkroin PA, editor. Encyclopedia of basic epilepsy research. 1st ed. Cambridge University Press.
Reinhart CJ, McIntyre DC, Pellis SM, Kolb BE. Prefrontal neuronal morphology in kindling-prone (FAST) and kindling-resistant (SLOW) rats. Synapse. 2021;75(9): e22217. https://doi.org/10.1002/syn.22217.
Kücker S, Töllner K, Piechotta M, Gernert M. Kindling as a model of temporal lobe epilepsy induces bilateral changes in spontaneous striatal activity. Neurobiol Dis. 2010;37(3):661–72. https://doi.org/10.1016/j.nbd.2009.12.002.
Funding
The study was supported by Grant 2018/28/C/NZ7/00240 from the National Science Centre in Poland.
Author information
Authors and Affiliations
Contributions
Aleksandra Wisłowska-Stanek: conceptualization, formal analysis, original draft writing. Danuta Turzyńska: conceptualization, behavioral tests, HPLC analysis. Alicja Sobolewska: statistical analysis, HPLC analysis. Anna Skórzewska: statistical analysis, validation. Karolina Kołosowska: methodology, validation, writing—review and editing. Janusz Szyndler: conceptualization, writing—review and editing. Piotr Maciejak: methodology, data analysis, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The study was approved by the Committee for Animal Care and Use at the Medical University in Warsaw (Consent number 29/2010) and was consistent with the European Communities Council Directive (2010/63/EU).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wisłowska-Stanek, A., Turzyńska, D., Sobolewska, A. et al. The effect of valproate on the amino acids, monoamines, and kynurenic acid concentrations in brain structures involved in epileptogenesis in the pentylenetetrazol-kindled rats. Pharmacol. Rep 76, 348–367 (2024). https://doi.org/10.1007/s43440-024-00573-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-024-00573-w