Abstract
The discovery of the glymphatic system has fundamentally altered our comprehension of cerebrospinal fluid transport and the removal of waste from brain metabolism. In the past decade, since its initial characterization, research on the glymphatic system has surged exponentially. Its potential implications for central nervous system disorders have sparked significant interest in the field of neurosurgery. Nonetheless, ongoing discussions and debates persist regarding the concept of the glymphatic system, and our current understanding largely relies on findings from experimental animal studies. This review aims to address several key inquiries: What methodologies exist for evaluating glymphatic function in humans today? What is the current evidence supporting the existence of a human glymphatic system? Can the glymphatic system be considered distinct from the meningeal-lymphatic system? What is the human evidence for glymphatic-meningeal lymphatic system failure in neurosurgical diseases? Existing literature indicates a paucity of techniques available for assessing glymphatic function in humans. Thus far, intrathecal contrast-enhanced magnetic resonance imaging (MRI) has shown the most promising results and have provided evidence for the presence of a glymphatic system in humans, albeit with limitations. It is, however, essential to recognize the interconnection between the glymphatic and meningeal lymphatic systems, as they operate in tandem. There are some human studies demonstrating deteriorations in glymphatic function associated with neurosurgical disorders, enriching our understanding of their pathophysiology. However, the translation of this knowledge into clinical practice is hindered by the constraints of current glymphatic imaging modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery of the glymphatic system in 2012 [34] sparked a significant shift in our understanding of cerebrospinal fluid (CSF) dynamics and its crucial role in clearing waste from the brain. Over the past few years, there has been a substantial increase in the literature on experimental studies in animals, particularly rodents, with broad implications for the treatment of central nervous system (CNS) diseases (for review see [57]).
The glymphatic system encompasses a brain-wide perivascular transport pathway for fluids and solutes, believed to be pivotal in removing metabolic waste from the brain [52], while also facilitating the transportation of substances to the brain [44]. In rodents, the glymphatic system is by far most active during sleep [79], but its efficacy diminishes with aging [41] and in systemic diseases such as experimental arterial hypertension [48] and diabetes [36]. Moreover, impaired glymphatic function may associate with the accumulation of toxic waste, including amyloid-β, tau, and α-synuclein in the brain, suggesting a significant role in dementia diseases like Alzheimer’s and Parkinson’s [7, 52]. It has also been proposed to play a crucial role in brain edemas resulting from stroke [49] and traumatic brain injury [33].
The glymphatic system has garnered attention in neurosurgical literature, with expectations regarding its potential impact on neurosurgical practices [2, 72]. However, critics highlight unresolved and debated aspects of the glymphatic concept [30, 50]. It is worth noting that the bulk of research on the glymphatic system has been conducted in animals, leaving questions unanswered regarding its translation to humans. From a clinical standpoint, the relevance of the glymphatic system depends on our ability to measure its function or dysfunction, as well as to identify changes in glymphatic function in response to interventions.
Against this backdrop, this review critically examines the following questions: (1) What methodologies exist for evaluating glymphatic function in humans today? (2) What is the current evidence supporting the existence of a human glymphatic system? (3) Can the glymphatic system be considered distinct from the meningeal-lymphatic system? (4) What is the human evidence for glymphatic-meningeal lymphatic system failure in neurosurgical diseases?
What methodologies exist for evaluating glymphatic function in humans today?
Today, the methods for assessing glymphatic function in humans predominantly hinge on magnetic resonance imaging (MRI) [40, 70]. More modalities are available in animals but are not commented on here. Table 1 provides an overview of the currently used human methods, each with its own set of advantages and disadvantages.
Intrathecal contrast-enhanced MRI
The initial demonstration occurred in a patient investigated for potential CSF leakage, where intrathecally administered gadobutrol (Gadovist, Bayer, GE) enriched brain tissue [15], indicating the free passage of the contrast agent from the subarachnoid space to the cerebral cortex and subcortical white matter. Subsequently, it was revealed that intrathecal gadobutrol enriches the entire brain in a centripetal manner, moving from the cortical surface inward [62, 63, 77]. The extent of tracer enrichment heavily relies on the amount of tracer in the subarachnoid CSF. Drawbacks include the requirement for spinal puncture and the off-label use of gadobutrol for intrathecal administration, which raises concerns about potential toxic effects and brain deposition. However, these concerns may be overstated for several reasons: (a) Toxic effects have not been observed in hundreds of patients receiving intrathecal gadobutrol in doses of 0.25 to 0.50 mmol [12, 28, 69]. (b) Gadobutrol retention in the human brain was not evident after four weeks [64]. (c) Considering that the on-label dosage of intravenous gadobutrol is significantly higher than the intrathecal dosage, CSF concentrations are comparable following intrathecal and intravenous injections [74].
Intravenous contrast-enhanced MRI
Due to the necessity of spinal puncture in intrathecal contrast-enhanced MRI, researchers have explored the visualization of glymphatic transport using intravenous contrast agents [81]. The concept is that some contrast enters the CSF, allowing for the evaluation of extravascular transport. However, a major drawback is the difficulty in distinguishing between glymphatic and vascular tracer enrichment since contrast may also leak from blood through the blood-brain-barrier.
MRI diffusion tensor image analysis along the perivascular space (DTI-ALPS)
A widely used non-invasive MRI method for glymphatic visualization is diffusion MRI, particularly the DTI-ALPS technique [71]. Despite its increasing popularity, this method has significant limitations [58]: (a) It measures water diffusivity in deep white matter, whereas glymphatic function pertains to solute and fluid transport rather than water transport alone. (b) Events in deep white matter may offer limited insight into glymphatic function, which is primarily a cortical phenomenon. (c) The vasculature in deep white matter and cerebral cortex differs. (d) The perivascular spaces encompass less than 1% of the brain volume [4], and the DTI-ALPS region of interest may not isolate water motion in the perivascular space from other directional water transport in white matter, for instance along axons. Consequently, there are substantial concerns regarding the use of DTI-ALPS as a measure of glymphatic function.
Perivascular spaces (PVS) of deep white matter
Another imaging option involves assessing enlarged white matter PVSs as non-invasive measure of glymphatic function [76]. The burden of enlarged PVS in the centrum semiovale and basal ganglia have been proposed as potential non-invasive measures of glymphatic function [53]. However, concerns remain regarding the communication between white matter PVS and CSF, as well as the relationship between events in white matter and the cerebral cortex. There may also exist other confounding factors behind enlarged PVS rather than impaired glymphatic function.
Magnetic resonance encephalography (MREG)
Another non-invasive approach to evaluate glymphatic function is ultra-fast MREG [39], which non-invasively assesses three types of physiological measures affecting brain pulsations (cardiac, respiratory and slow waves). While being a promising non-invasive technique, providing for unique insights into brain pulsations, the primary challenge lies in determining the extent to which alterations observed relate to changes in glymphatic function.
Overall, there is currently a scarcity of methods for clinically assessing glymphatic function in humans. Presently, intrathecal contrast-enhanced MRI, as developed by the author and colleagues, is by several considered the gold standard for glymphatic imaging in humans [74].
What is the current evidence supporting the existence of a human glymphatic system?
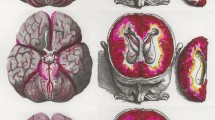
The current evidence supporting the existence of a human glymphatic system heavily relies on observations made through intrathecal contrast-enhanced MRI, where the contrast agent acts as a CSF tracer. Some principal lines of evidence are depicted in Fig. 1 and can be summarized as follows:
Glymphatic enrichment of a CSF tracer in a human subject is depicted in this figure. Currently, intrathecal contrast-enhanced MRI is considered the gold standard for glymphatic imaging in humans. Following the intrathecal injection of a CSF tracer, such as gadobutrol (Gadovist, Bayer, GE; 0.50 mmol, total volume 1 ml), tracer enrichment is visualized using standardized MRI T1 acquisitions, as previously described. The tracer first enriches the subarachnoid spaces (A), then progresses to the cerebral cortex and subcortical white matter (B), as indicated by the percentage increase on the color bars to the right at 7 h. It’s worth noting that the strongest enrichment within the subarachnoid space (A) corresponds to the area of strongest enrichment in the cerebral cortex (B). By 24 h, tracer enrichment remained comparable in the subarachnoid space but increased in the cerebral ventricles (C), while glymphatic enrichment became brain-wide at this time (D; percentage increase in tracer enrichment shown on the color bars to the right). The tracer gadobutrol is hydrophilic and does not pass the blood-brain barrier; instead, it remains confined to the extravascular compartment when administered intrathecally. It is a neutral compound with a molecular weight of 604 Da. Images provided by Lars Magnus Valnes, PhD, Department of Neurosurgery, Oslo University Hospital-Rikshospitalet
Brain-wide distribution of a CSF tracer
The glymphatic system functions as a brain-wide perivascular transport network for fluids and solutes, featuring periarterial influx and perivenous outflux pathways. The contrast agent, acting as a CSF tracer, initially enriches the subarachnoid CSF space and subsequently permeates the entire brain in a centripetal manner, from the cortical surface to subcortical regions [63]. Given the typical 1 mm resolution of MRI, the precise route of tracer passage - whether perivascular along arteries or veins, along the basement membrane of capillaries, or across the pia mater into the interstitial tissue - remains undetermined. Evidence supporting periarterial tracer passage along vessels includes tracer enrichment in the cerebral cortex adjacent to major artery trunks of the subarachnoid space, such as the anterior cerebral artery, middle cerebral artery, and posterior cerebral artery [17, 63]. Importantly, studies administering a CSF tracer to the subarachnoid space of pigs yielded comparable tracer distribution patterns as observed in humans, with immunohistochemistry and microscopic examinations confirming tracer confinement to the perivascular spaces [5]. A notable observation from human studies is that significant, inter-individual enrichment patterns in brain exist [63] (see Fig. 2).
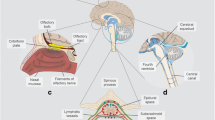
The parasagittal dura. An MRI contrast agent utilized as a cerebrospinal fluid (CSF) tracer enriches the CSF within the subarachnoid space (SAS) and traverses the arachnoid membrane into the dura mater along the superior sagittal sinus (SSS), known as the parasagittal dura (PSD). This phenomenon is depicted here through 3D images generated from T2-FLAIR images, co-registered with brain segmentation and CSF tracer enhancement from T1 GRE at 48 h post-intrathecal tracer injection. (A) The superior sagittal sinus (SSS) is highlighted in blue, while the parasagittal dura (PSD) is depicted in yellow. (B) The PSD may extend into the marrow of the skull bone (SB). Additionally, a vein (V) within the diploic space is shown. CC: Cerebral cortex. Images by Tomas Sakinis, MD, Department of Radiology, Oslo University Hospital-Rikshospitalet.
Tracer distribution in the human brain compared with the pattern of toxic metabolite accumulation in dementia diseases
The glymphatic system is hypothesized to serve as a clearance pathway for toxic metabolites like amyloid-β, tau in Alzheimer’s disease, and α-synuclein in Parkinson’s disease. The pathological aggregation of these metabolites in dementia diseases follows a characteristic pattern, which aligns to some degree with the distribution of tracer observed in human studies [52].
Tracer transport not solely explained by diffusion
The glymphatic concept suggests that perivascular solute transport relies on convective forces (i.e., pressure-gradient forces), with diffusion potentially more prominent in the interstitial tissue. In humans, brain-wide tracer transport occurs over hours [63], a prolonged phenomenon not solely explained by diffusion, indicating the involvement of additional forces [73, 75].
Facilitated solute transport along subarachnoid perivascular subarachnoid spaces (PVSAS)
Subpial periarterial influx of CSF constitutes a crucial aspect of the glymphatic system [34]. In humans, facilitated tracer transport occurs along major cerebral vessels anterior cerebral artery, middle cerebral artery and posterior cerebral artery within the subarachnoid space, followed by enrichment of the cerebral cortex where arteries penetrate the brain [17]. This facilitates the antegrade transport of fresh CSF along arteries towards the brain within the PVSAS, while CSF containing waste products empties perivenously into the subarachnoid space outside PVSAS, to be expelled from the subarachnoid space at arachnoid cuff exit (ACE) points [68].
CSF tracer dynamics from the human brain is sleep-dependent
In mice, the glymphatic system primarily operates during sleep [79], whereas in humans, clearance of tracer from the cerebral cortex and subcortical white matter significantly decreases after one night of total sleep deprivation [20], albeit to a lesser extent than observed in animals. One night of sleep deprivation also increased accumulation of amyloid-β in the hippocampus and thalamus of healthy volunteers [67]. Modeling studies also suggest a weaker effect of sleep deprivation on tracer clearance in humans than rodents, though the impact in humans remains demonstrable [75]. Furthermore, in patients with chronic impaired sleep quality, tracer enrichment and clearance in the human brain become altered [23].
Association between markers of glymphatic function and plasma biomarkers of dementia
The glymphatic system’s primary function is proposed to be the clearance of toxic waste products from brain metabolism, with impaired glymphatic clearance hypothesized to underlie the abnormal aggregation of toxic waste seen in dementia diseases. In humans, markers of glymphatic function derived from CSF tracer assessments correlate with plasma biomarkers of neurodegeneration [24].
Role of the water channel aquaporin-4 (AQP4) for glymphatic transport in humans
Indirect evidence suggests a potential role of AQP4 in glymphatic transport in humans. Cortical biopsies from patients with idiopathic normal pressure hydrocephalus (iNPH) demonstrate loss of perivascular AQP4 [13, 29]. The iNPH patients also show impaired glymphatic enrichment [63]. However, further investigation is required to determine whether the loss of perivascular AQP4 is a causative mechanism behind the glymphatic failure. In this regard, it is worth noting that a recent study found that acute treatment with the AQP4 inhibitor AER-271 inhibited glymphatic flow in mice, without altering the localization of AQP4 to astrocytic endfeet [26].
Evidence of impaired glymphatic clearance in patients
Evidence for a human glymphatic system also relies on the in vivo evidence for impaired glymphatic clearance in patients with iNPH [16, 63] and idiopathic intracranial hypertension (IIH) [21].
In summary, the evidence supporting the existence and function of the human glymphatic system is multi-faceted, encompassing various lines of inquiry and observations.
Can the glymphatic system be considered distinct from the meningeal-lymphatic system?
In addition to the discovery of the glymphatic system, the rediscovery of meningeal lymphatic vessels capable of draining CSF to dural and extra-dural lymphatic structures represented a breakthrough [3, 45]. The meningeal lymphatic pathways may serve as a final common pathway for the clearance of substances from both the glymphatic pathways and the CSF; its function impairs with age [65]. While the glymphatic system is a brain-wide clearance system involving the CSF, current understanding indicates that clearance primarily occurs to the subarachnoid CSF. The subsequent step involves clearance from the CSF, a process not fully explained by the glymphatic system. Obstruction of this clearance route may exacerbate waste accumulation (including amyloid-β, tau and α-synuclein) and dementia disease progression [9, 10, 54]. Therefore, the glymphatic system should not be viewed in isolation from the meningeal lymphatic system. Additionally, the meningeal lymphatic system plays a crucial role in CNS immunosurveillance, which may significantly impact the glymphatic system. In this regard, it is worth noting that perivascular macrophages play an important role in clearing the perivascular pathways [11].
In human tracer studies, it was observed that tracer in the subarachnoid CSF passed directly to the parasagittal dura (Fig. 2) through the arachnoid membrane (although the exact site of transport was not defined) [59], the marrow of skull bone [60], and even to extracranial lymph nodes [18]. A significant observation is that the amount of tracer in the subarachnoid CSF determines the extent of tracer enrichment in the parasagittal dura as well as in the brain [59, 62]. Therefore, the CSF in the subarachnoid space seems to serve as a reservoir for metabolites, from which substances are transported via lymphatic dural vessels to peripheral lymph nodes and blood.
However, there has been controversy regarding how substances within the subarachnoid CSF are transported to the dura mater, considering the barrier properties of the arachnoid barrier cell layer [78]. A recent significant discovery was the identification of openings in the arachnoid barrier cell layer where bridging veins pass from the cerebral cortex to the dura mater; these openings were delineated by arachnoid cuffs, creating arachnoid cuff exit (ACE) points in the arachnoid where cells and substances may pass along the perivenous basement membrane toward the dura mater [68]. Passage of cells and substances also occurred from outside to CSF.
Imaging the capacity of meningeal lymphatic clearance can pose challenges [61]. Thus, we propose evaluating from plasma samples the CSF-to-blood clearance of an intrathecal tracer, as a surrogate marker of meningeal lymphatic clearance capacity [22]. Pharmacokinetic modeling allows for determining individual CSF-to-blood clearance capacities, revealing significant inter-individual variability [31]. Just as the dose of intravenous drugs can be tailored based on renal clearance function, as measured by the glomerular filtration rate (GFR), so too can the dose of intrathecal drugs be adjusted based on CSF-to-blood clearance function.
It’s worth noting that the primary route for CSF efflux predominantly takes place at the spinal level. Studies showed that peak plasma levels of CSF tracer [31] are observed several hours prior to the peak enrichment of the tracer in the PSD [59]. Modeling studies have further suggested that approximately two-thirds of the total CSF efflux transpires from the spinal canal [75]. Additionally, CSF efflux at the skull base could also be significant, as previously demonstrated experimentally [1].
In summary, in the context of brain clearance, it may be more useful to consider the glymphatic-meningeal lymphatic system as interconnected entities.
What is the human evidence for glymphatic-meningeal lymphatic system failure in neurosurgical diseases?
In the neurosurgical community, there is a growing awareness of the potential implications of glymphatic failure for neurosurgical diseases [2, 72]. This relates to burgeoning body of experimental literature suggesting a role of glymphatic dysfunction in conditions such as edema following subarachnoid hemorrhage [8, 25], traumatic brain injury [6, 33, 35], post-stroke edema [49], post-hemicraniectomy features [56], subdural hematoma [43, 66], and primary brain tumors [32, 46].
However, the focus of this review is not on experimental animal studies but rather on the human evidence for glymphatic failure in neurological disorders.
Idiopathic normal pressure hydrocephalus (iNPH)
This disease stands out as the most extensively studied condition to date. In iNPH, the perivascular spaces of the subarachnoid space (PVSAS) exhibit dysfunction, characterized by widened PVSAS areas and slowed perivascular tracer transport [17]. This is accompanied with enhanced tracer enrichment in the brain and slowed clearance, likely due to impaired glymphatic transport. Notably, this impairment is evident in the entorhinal cortex [16], a region critical for cognitive function [51], suggesting potential clinical relevance to the cognitive decline observed in iNPH patients. Furthermore, this patient group demonstrates pronounced ventricular tracer enrichment caused by tracer reflux into the ventricles [19, 62, 63]. These findings indicate marked alterations in solute transport within the CSF in iNPH, which may contribute to the accumulation of amyloid-β and tau in the cerebral cortex of these patients [42]. The iNPH disease should be considered a combined neurodegenerative and CSF disease where the shunt surgery mainly addresses the CSF component.
Idiopathic intracranial hypertension (IIH)
The IIH patients also exhibit evidence of delayed brain-wide tracer clearance [21]. This is of interest given that IIH patients may present with cognitive impairment [80]. Additionally, this patient group presents with an increased number of enlarged white matter perivascular spaces in the centrum semiovale and basal ganglia [37]. While IIH has traditionally been viewed as a CSF or venous obstruction disease, observations of glymphatic failure suggest a more widespread brain effect, which may be interpreted as consistent with histopathological data [14].
Subarachnoid hemorrhage (SAH)
Following SAH, increased number of enlarged white matter perivascular spaces in the centrum semiovale was reported [38], which authors attribute to glymphatic failure. A previous non-human primate study also provided evidence of glymphatic dysfunction after SAH [27].
Traumatic brain injury (TBI)
Glymphatic function has to a lesser degree been studied in TBI patients, but recent experimental evidence suggests a crucial role of glymphatic function for brain edema [33]. In patients with traumatic brain injury (TBI), those with poor sleep quality exhibit evidence of enlarged white matter perivascular spaces [53]. Additionally, there was a significant positive correlation between the number and volume of these spaces and the number of previous mild TBIs, the severity of post-concussive symptoms, and post-traumatic balance issues [55].
Diseases affecting the spinal cord
While diseases affecting the spinal cord have not yet been extensively explored, there is evidence of strong glymphatic enrichment within the spinal cord [47].
Currently, the human evidence for glymphatic alterations in neurosurgical diseases remains limited. However, it is anticipated that this landscape will evolve with further research.
Future directions
Studies on glymphatic function in neurosurgical diseases have offered new insights into disease mechanisms, yet the assessment of glymphatic function in neurosurgical practice has been minimally implemented. To the best of our knowledge, the one example is use of intrathecal contrast-enhanced MRI in assessment of iNPH patients in our institution [19]. A clear objective for the future is the incorporation of methods for assessing glymphatic and meningeal lymphatic functions before, during, and after interventions. To effect change in neurosurgical practice, the evaluation of glymphatic function must be integrated into treatments or interventions, possibly even on multiple occasions. However, this currently poses a challenge due to the limited availability of methods.
Another crucial goal should be individualized assessments, considering the significant inter-individual variation observed both in glymphatic tracer enrichment in the human brain [63] and in CSF-to-blood clearance [31].
Conclusion
The discovery of the glymphatic system has sparked a paradigm shift in our comprehension of the role of CSF in CNS function, with growing recognition of potential implications in neurosurgical diseases. While the bulk of research originates from experimental studies, this review has concentrated on evidence gleaned from human studies. Undoubtedly, there is a dearth of methodologies suitable for studying glymphatic function in humans. Intrathecal contrast-enhanced MRI was initially introduced and remains the most valuable methodology, albeit with limitations. There is an imperative need for overcoming these imaging obstacles. Despite these limitations, several lines of evidence suggest the presence of a human glymphatic system that may falter in neurosurgical diseases. However, to impact neurosurgical practice, clinically available tools are required to assess glymphatic and meningeal lymphatic function.
Abbreviations
- ACE:
-
Arachnoid cuff exits
- AQP4:
-
Aquaporin-4
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DTI-ALPS:
-
Diffusion tensor image analysis along the perivascular space
- GFR:
-
Glomerular filtration rate
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- IIH:
-
Idiopathic intracranial hypertension
- MREG:
-
Magnetic resonance encephalography
- MRI:
-
Magnetic resonance imaging
- PSD:
-
Parasagittal dura
- PVS:
-
Perivascular space
- PVSAS:
-
Perivascular subarachnoid space
- SAH:
-
Subarachnoid hemorrhage
- TBI:
-
Traumatic brain injury
References
Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, Jeong Y, Park SH, Koh GY (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572:62–66
Al Masri M, Corell A, Michaëlsson I, Jakola AS, Skoglund T (2024) The glymphatic system for neurosurgeons: a scoping review. Neurosurg Rev 47:61
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991–999
Barisano G, Sheikh-Bahaei N, Law M, Toga AW, Sepehrband F (2021) Body mass index, time of day and genetics affect perivascular spaces in the white matter. J Cereb Blood Flow Metab 41:1563–1578
Bèchet NB, Shanbhag NC, Lundgaard I (2021) Glymphatic pathways in the gyrencephalic brain. J Cereb Blood Flow Metab 41:2264–2279
Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, Ennerfelt HE, Shapiro D, Nguyen BH, Frost EL, Lammert CR, Kipnis J, Lukens JR (2020) Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun 11:4524
Buccellato FR, D’Anca M, Serpente M, Arighi A, Galimberti D (2022) The Role of Glymphatic System in Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines 10
Chen J, Wang L, Xu H, Xing L, Zhuang Z, Zheng Y, Li X, Wang C, Chen S, Guo Z, Liang Q, Wang Y (2020) Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun 11:3159
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J (2018) Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560:185–191
Ding XB, Wang XX, Xia DH, Liu H, Tian HY, Fu Y, Chen YK, Qin C, Wang JQ, Xiang Z, Zhang ZX, Cao QC, Wang W, Li JY, Wu E, Tang BS, Ma MM, Teng JF, Wang XJ (2021) Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat Med 27:411–418
Drieu A, Du S, Kipnis M, Bosch ME, Herz J, Lee C, Jiang H, Manis M, Ulrich JD, Kipnis J, Holtzman DM, Gratuze M (2023) Parenchymal border macrophages regulate tau pathology and tau-mediated neurodegeneration. Life Sci Alliance 6
Edeklev CS, Halvorsen M, Lovland G, Vatnehol SAS, Gjertsen O, Nedregaard B, Sletteberg R, Ringstad G, Eide PK (2019) Intrathecal Use of Gadobutrol for Glymphatic MR Imaging: prospective safety study of 100 patients. AJNR Am J Neuroradiol 40:1257–1264
Eide PK, Hansson HA (2018) Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol Appl Neurobiol 44:474–490
Eide PK, Hansson HA (2022) A New Perspective on the pathophysiology of idiopathic intracranial hypertension: role of the glia-neuro-vascular interface. Front Mol Neurosci 15:1–24
Eide PK, Ringstad G (2015) MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol open 4:1–5
Eide PK, Ringstad G (2019) Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab 39:1355–1368
Eide PK, Ringstad G (2024) Functional analysis of the human perivascular subarachnoid space. Nat Commun 15:2001
Eide PK, Vatnehol SAS, Emblem KE, Ringstad G (2018) Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep 8:1–10
Eide PK, Pripp AH, Ringstad G (2020) Magnetic resonance imaging biomarkers of cerebrospinal fluid tracer dynamics in idiopathic normal pressure hydrocephalus. Brain Commun 2:1–16
Eide PK, Vinje V, Pripp AH, Mardal KA, Ringstad G (2021) Sleep deprivation impairs molecular clearance from the human brain. Brain 144:863–874
Eide PK, Pripp AH, Ringstad G, Valnes LM (2021) Impaired glymphatic function in idiopathic intracranial hypertension. Brain Commun 3:1–14
Eide PK, Mariussen E, Uggerud H, Pripp AH, Lashkarivand A, Hassel B, Christensen H, Hovd MH, Ringstad G (2021) Clinical application of intrathecal gadobutrol for assessment of cerebrospinal fluid tracer clearance to blood. JCI Insight 6:1–13
Eide PK, Pripp AH, Berge B, Hrubos-Strøm H, Ringstad G, Valnes LM (2022) Altered glymphatic enhancement of cerebrospinal fluid tracer in individuals with chronic poor sleep quality. J Cereb Blood Flow Metab 42:1676–1692
Eide PK, Lashkarivand A, Pripp A, Valnes LM, Hovd MH, Ringstad G, Blennow K, Zetterberg H (2023) Plasma neurodegeneration biomarker concentrations associate with glymphatic and meningeal lymphatic measures in neurological disorders. Nat Commun 14:2084
Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M (2014) Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 45:3092–3096
Giannetto MJ, Gomolka RS, Gahn-Martinez D, Newbold EJ, Bork PAR, Chang E, Gresser M, Thompson T, Mori Y, Nedergaard M (2024) Glymphatic fluid transport is suppressed by the aquaporin-4 inhibitor AER-271. Glia
Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, Gakuba C, Emery E, Hantraye P, Vivien D, Aron-Badin R, Gaberel T (2017) Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in Nonhuman Primate. Stroke 48:2301–2305
Halvorsen M, Edeklev CS, Fraser-Green J, Lovland G, Vatnehol SAS, Gjertsen O, Nedregaard B, Sletteberg R, Ringstad G, Eide PK (2021) Off-label intrathecal use of gadobutrol: safety study and comparison of administration protocols. Neuroradiology 63:51–61
Hasan-Olive MM, Enger R, Hansson HA, Nagelhus EA, Eide PK (2019) Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia 67:91–100
Hladky SB, Barrand MA (2022) The glymphatic hypothesis: the theory and the evidence. Fluids Barriers CNS 19:9
Hovd MH, Mariussen E, Uggerud H, Lashkarivand A, Christensen H, Ringstad G, Eide PK (2022) Population pharmacokinetic modeling of CSF to blood clearance: prospective tracer study of 161 patients under work-up for CSF disorders. Fluids Barriers CNS 19:55
Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, He T, Ning W, Kong Y, Huo Y, He A, Liu B, Zhang J, Adams R, He Y, Tang F, Bian X, Luo J (2020) Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 30:229–243
Hussain R, Tithof J, Wang W, Cheetham-West A, Song W, Peng W, Sigurdsson B, Kim D, Sun Q, Peng S, Plá V, Kelley DH, Hirase H, Castorena-Gonzalez JA, Weikop P, Goldman SA, Davis MJ, Nedergaard M (2023) Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature 623:992–1000
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34:16180–16193
Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z (2017) Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 37:1326–1337
Jones O, Cutsforth-Gregory J, Chen J, Bhatti MT, Huston J, Brinjikji W (2021) Idiopathic intracranial hypertension is Associated with a higher burden of visible cerebral perivascular spaces: the glymphatic connection. AJNR Am J Neuroradiol 42:2160–2164
Kim J, Joo B, Kim JW, Park M, Ahn SJ, Park SK, Suh SH (2022) Aggravation of Enlarged Perivascular spaces in the Centrum Semiovale of patients with Aneurysmal Subarachnoid Hemorrhage. Clin Neuroradiol 32:79–87
Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M (2016) Ultra-fast magnetic resonance encephalography of physiological brain activity - glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab 36:1033–1045
Klostranec JM, Vucevic D, Bhatia KD, Kortman HGJ, Krings T, Murphy KP, terBrugge KG, Mikulis DJ (2021) Current Concepts in Intracranial Interstitial Fluid Transport and the Glymphatic System: Part II-Imaging Techniques and Clinical Applications. Radiology:204088
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861
Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Tamminen JN, Tillgren T, Vainikka S, Pyykko OT, Molsa J, Fraunberg M, Pirttila T, Jaaskelainen JE, Soininen H, Rinne J, Alafuzoff I (2010) Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann Neurol 68:446–453
Liu X, Gao C, Yuan J, Xiang T, Gong Z, Luo H, Jiang W, Song Y, Huang J, Quan W, Wang D, Tian Y, Ge X, Lei P, Zhang J, Jiang R (2020) Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun 8:16
Lohela TJ, Lilius TO, Nedergaard M (2022) The glymphatic system: implications for drugs for central nervous system diseases. Nat Rev Drug Discov 21:763–779
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341
Ma Q, Schlegel F, Bachmann SB, Schneider H, Decker Y, Rudin M, Weller M, Proulx ST, Detmar M (2019) Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci Rep 9:14815
Melin E, Pripp AH, Eide PK, Ringstad G (2023) In vivo distribution of cerebrospinal fluid tracer in human upper spinal cord and brain stem. JCI Insight 8
Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9:4878
Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, Mortensen KN, Staeger FF, Bork PAR, Bashford L, Toro ER, Tithof J, Kelley DH, Thomas JH, Hjorth PG, Martens EA, Mehta RI, Solis O, Blinder P, Kleinfeld D, Hirase H, Mori Y, Nedergaard M (2020) Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367
Mestre H, Mori Y, Nedergaard M (2020) The Brain’s Glymphatic System: current controversies. Trends Neurosci 43:458–466
Moser MB, Rowland DC, Moser EI (2015) Place cells, grid cells, and memory. Cold Spring Harb Perspect Biol 7:a021808
Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370:50–56
Opel RA, Christy A, Boespflug EL, Weymann KB, Case B, Pollock JM, Silbert LC, Lim MM (2019) Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J Cereb Blood Flow Metab 39:2258–2267
Patel TK, Habimana-Griffin L, Gao X, Xu B, Achilefu S, Alitalo K, McKee CA, Sheehan PW, Musiek ES, Xiong C, Coble D, Holtzman DM (2019) Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegeneration 14:11
Piantino J, Lim MM, Newgard CD, Iliff J (2019) Linking traumatic Brain Injury, Sleep disruption and post-traumatic headache: a potential role for glymphatic pathway dysfunction. Curr Pain Headache Rep 23:62
Plog BA, Lou N, Pierre CA, Cove A, Kenney HM, Hitomi E, Kang H, Iliff JJ, Zeppenfeld DM, Nedergaard M, Vates GE (2019) When the air hits your brain: decreased arterial pulsatility after craniectomy leading to impaired glymphatic flow. J Neurosurg :1–14
Rasmussen MK, Mestre H, Nedergaard M (2022) Fluid transport in the brain. Physiol Rev 102:1025–1151
Ringstad G (2024) Glymphatic imaging: a critical look at the DTI-ALPS index. Neuroradiology 66:157–160
Ringstad G, Eide PK (2020) Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun 11:354
Ringstad G, Eide PK (2022) Molecular trans-dural efflux to skull bone marrow in humans with CSF disorders. Brain 145:1464–1472
Ringstad G, Eide PK (2024) Glymphatic-lymphatic coupling: assessment of the evidence from magnetic resonance imaging of humans. Cell Mol Life Sci 81:131
Ringstad G, Vatnehol SAS, Eide PK (2017) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140:2691–2705
Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, Mardal KA, Eide PK (2018) Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3:1–16
Ringstad G, Valnes LM, Vatnehol SAS, Pripp AH, Eide PK (2023) Prospective T1 mapping to assess gadolinium retention in brain after intrathecal gadobutrol. Neuroradiology
Rustenhoven J, Pavlou G, Storck SE, Dykstra T, Du S, Wan Z, Quintero D, Scallan JP, Smirnov I, Kamm RD, Kipnis J (2023) Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J Exp Med 220
Shanbhag NC, Bèchet NB, Kritsilis M, Lundgaard I (2021) Impaired cerebrospinal fluid transport due to idiopathic subdural hematoma in pig: an unusual case. BMC Vet Res 17:250
Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, De Santi S, Tomasi D, Benveniste H, Volkow ND (2018) β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. U. S. A. 115:4483–4488
Smyth LCD, Xu D, Okar SV, Dykstra T, Rustenhoven J, Papadopoulos Z, Bhasiin K, Kim MW, Drieu A, Mamuladze T, Blackburn S, Gu X, Gaitán MI, Nair G, Storck SE, Du S, White MA, Bayguinov P, Smirnov I, Dikranian K, Reich DS, Kipnis J (2024) Identification of direct connections between the dura and the brain. Nature 627:165–173
Sperre A, Karsrud I, Rodum AHS, Lashkarivand A, Valnes LM, Ringstad G, Eide PK (2023) Prospective Safety Study of Intrathecal Gadobutrol in different doses. AJNR Am J Neuroradiol 44:511–516
Taoka T, Naganawa S (2020) Glymphatic imaging using MRI. J Magn Reson Imaging 51:11–24
Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, Kishimoto T, Naganawa S (2017) Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 35:172–178
Treffy RW, Eraky AM, Hussain O, Hedayat Hs (2023) Glymphatics for the neurosurgeon. Neurosurg Pract 4:1–5
Valnes LM, Mitusch SK, Ringstad G, Eide PK, Funke SW, Mardal KA (2020) Apparent diffusion coefficient estimates based on 24 hours tracer movement support glymphatic transport in human cerebral cortex. Sci Rep 10:1–12
van Osch MJP, Wåhlin A, Scheyhing P, Mossige I, Hirschler L, Eklund A, Mogensen K, Gomolka R, Radbruch A, Qvarlander S, Decker A, Nedergaard M, Mori Y, Eide PK, Deike K, Ringstad G (2024) Human brain clearance imaging: pathways taken by magnetic resonance imaging contrast agents after administration in cerebrospinal fluid and blood. NMR Biomed.:e5159
Vinje V, Zapf B, Ringstad G, Eide PK, Rognes ME, Mardal KA (2023) Human brain solute transport quantified by glymphatic MRI-informed biophysics during sleep and sleep deprivation. Fluids Barriers CNS 20:62
Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE (2020) Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Reviews Neurol 16:137–153
Watts R, Steinklein JM, Waldman L, Zhou X, Filippi CG (2019) Measuring Glymphatic Flow in Man using quantitative contrast-enhanced MRI. AJNR Am J Neuroradiol 40:648–651
Weller RO, Sharp MM, Christodoulides M, Carare RO, Møllgård K (2018) The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol 135:363–385
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377
Yri HM, Fagerlund B, Forchhammer HB, Jensen RH (2014) Cognitive function in idiopathic intracranial hypertension: a prospective case-control study. BMJ open 4:e004376
Zhang M, Tang J, Xia D, Xue Y, Ren X, Huang Q, Shi L, Tang W, Fu J (2023) Evaluation of glymphatic-meningeal lymphatic system with intravenous gadolinium-based contrast-enhancement in cerebral small-vessel disease. Eur Radiol 33:6096–6106
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eide, P.K. Neurosurgery and the glymphatic system. Acta Neurochir 166, 274 (2024). https://doi.org/10.1007/s00701-024-06161-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-06161-4