Abstract
Background
In neurosurgical patients, the risk of developing venous thromboembolism (VTE) is high due to the relatively long duration of surgical interventions, usually long immobilization time after surgery, and possible neurological deficits which can negatively influence mobility. In neurosurgical clinical practice, there is lack of consensus on optimal prophylaxis against VTE, mechanical or pharmacological.
Objective
To systematically review available literature on the incidence of VTE in neurosurgical interventions and to establish an optimum prevention strategy.
Methods
A literature search was performed in PubMed, Embase, Web of Science, Cochrane Library, and EmCare, based on a sensitive search string combination. Studies were selected by predefined selection criteria, and risk of bias was assessed by Newcastle–Ottawa Quality Assessment Scale and Cochrane risk of bias.
Results
Twenty-five studies were included, half of which had low risk of bias (21 case series, 3 comparative studies, 1 RCT). VTE was substantially higher if the evaluation was done by duplex ultrasound (DUS), or another systematic screening method, in comparison to clinical evaluation (clin). Without prophylaxis DVT, incidence varied from 4 (clin) to 10% (DUS), studies providing low molecular weight heparin (LMWH) reported an incidence of 2 (clin) to 31% (DUS), providing LMWH and compression stockings (CS) reported an incidence of 6.4% (clin) to 29.8% (DUS), and providing LMWH and intermittent pneumatic compression devices (IPC) reported an incidence of 3 (clin) to 22.3% (DUS). Due to a lack of data, VTE incidence could not meaningfully be compared between patients with intracranial and spine surgery. The reported incidence of pulmonary embolism (PE) was 0 to 7.9%.
Conclusion
Low molecular weight heparin, compression stockings, and intermittent pneumatic compression devices were all evaluated to give reduction in VTE, but data were too widely varying to establish an optimum prevention strategy. Systematic screening for DVT reveals much higher incidence percentages in comparison to screening solely on clinical grounds and is recommended in follow-up of neurosurgical procedures with an increased risk for DVT development in order to prevent occurrence of PE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thromboembolic prophylaxis is a crucial aspect of patient care in neurosurgical practice. Neurosurgical patients are at high risk for thromboembolic events, including deep vein thrombosis (DVT), pulmonary embolism (PE), and stroke. The risk of venous thromboembolic events (VTE) in neurosurgical patients is multifactorial, with several factors contributing to the development of these events [23]. One of the major contributors is the often occurring immobility during and bed rest after (lengthy) surgery, which can lead to venous stasis and impaired blood flow [27]. Other factors such as the use of vasopressors, dehydration, and motor deficits pre- and post-surgery also increase the risk of thromboembolic events [17, 23]. Finally, another factor that may lead to an increased risk of a thromboembolic event is a state of hypercoagulability, induced by the presence of malignant tumors or subarachnoid hemorrhage [44].
The clinically relevant symptoms associated with DVT are warmth, swelling, pain and redness of the leg, but the majority of VTE cases are asymptomatic [11, 17]. Meanwhile, the asymptomatic VTE localized in deep lower extremity veins may progress to symptomatic VTE [22, 28, 32, 37]. As for clinical examination, DVT can be confirmed by Doppler ultrasound (DUS) or venography [23, 45], and PE confirmed by computed tomography (CT) of the chest or angiography (CTA) [23, 45].
The CHEST Guidelines recommend that every hospital develops a formal strategy that addresses the prevention of VTE [15]. Thromboembolic prophylaxis includes pharmacological measures with usually low molecular weight heparin (LMWH) and non-pharmacological measures as early mobilization and physical therapy, compression stockings, and intermittent pneumatic compression (IPC) devices [15]. For “patients undergoing major neurosurgery,” the CHEST Guidelines recommend “optimal use of mechanical methods of thromboprophylaxis” with the acceptable alternative of LMWH. For this type of patients with a “particular high thrombosis risk,” it is recommended to combine mechanical prophylaxis with LMWH. In patients undergoing neurosurgery with high bleeding risk, the mechanical method is recommended to substitute LMWH [15]. Definitions are however not further specified.
Lack of knowledge on prophylaxis of thromboembolic events in neurosurgical clinical practice leads to absence of consensus on the choice of prophylaxis for VTE in the Netherlands. A wide diversity in choice (IPC, compression stockings, heparin, LMWH) and timing (preoperative, postoperative) of prophylactic measures was shown in an evaluation of the use of VTE prophylaxis in all seven university neurosurgical clinics in the Netherlands [26].
Previously, in 2012, we performed an extensive literature search on the incidence of thromboembolic events in patients undergoing spinal or intracranial neurosurgical procedures and summarized our results in a systematic review [26]. It was concluded that intracranial surgical patients were more at risk to develop a VTE compared to spinal surgery patients, that the use of antithrombotic prophylaxis in neurosurgical interventions lowers the VTE incidence from 30 to about 1.5 to 6%, that a twofold higher VTE rate was demonstrated in patients systematically screened for DVT in comparison to those solely clinically screened, and that subclinical DVT was described to be associated with the incidence of PE. However, large heterogeneity with respect to diagnostic methods for VTE events and variable antithrombotic prophylaxis prevented us from drawing firm conclusions on optimal treatment strategy. Now, 10 years later, we deemed it useful to perform an update of this review with the purpose of finding more definite answers to optimizing anti-thrombotic treatment strategy in neurosurgical patients.
Methods
Literature search strategy
A systematic review of the literature was performed by following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [31]. A comprehensive search strategy in PubMed, Embase, and Web of Science was executed to examine the incidence of VTE in the perioperative care in neurosurgery. The search strategy is shown in Appendix 1. Dates of the search queries included articles from October 2012 up to and including January 2022. This time course was chosen to expand beyond the literature search of our previous systematic review [26].
Eligibility criteria
Selection of articles was independently performed by two reviewers (ZZ and CV-L). The inclusion criteria were in line with the aforementioned review. Articles were considered eligible for inclusion if it concerned patients that underwent a neurosurgical intervention, if VTE was systematically evaluated and the method with which DVT and/or PE was diagnosed was clearly indicated. Both the nature of the intervention (intracranial or spinal intervention) and the anti-thrombotic prophylaxis (none, mechanical, chemical, or a combination) had to be clearly described. Case studies with a minimum of ten patients, cohort studies, and randomized controlled trials could be included; systematic reviews and meta-analyses were excluded.
After screening for eligibility according to the inclusion and exclusion criteria, the articles were further analyzed for relevance to determine the final article selection. Consensus about the selection was reached in open discussion.
Quality assessment
Three investigators (ZZ, HC, and CV-L) independently performed a risk of bias analysis by assessing the included observational cohort studies according to an adjusted Newcastle–Ottawa Scale (NOS) (Appendix 2) [46]. Additionally, the Cochrane risk of bias tool (Appendix 3) was used for comparative studies and randomized controlled studies. Any disagreements between the three investigators were resolved. Maximum scores were 4 for selection, 1 for comparability, and 4 for outcome assessment. The risk of bias was then ranked as high (≤ 2 points), moderate (3–4 points), or low (5 or more points) depending on the overall score. A score of 6 points or more (out of a maximum of 12 points) on the Cochrane risk of bias tool was defined as a low risk of bias.
Diagnosis of venous thromboembolism
DVT and PE can be diagnosed on clinical grounds. Symptoms of DVT include pain, swelling, redness, and warmth of the skin of the leg (usually the calf). The presence of symptoms can lead to a clinical diagnosis of DVT, which thereafter may or may not be confirmed with objective imaging or measurements. Symptoms of PE are pain in the thoracic area and dyspnoea. The clinical diagnosis of PE is generally followed by objective imaging evaluation due to its essential need for treatment.
DVT can also be systematically evaluated using a more objective screening method to evaluate the presence of DVT. This means that all participants in a study, regardless of whether or not they experience symptoms, will be evaluated using an objective screening method. The most common screening method to screen for DVT is by postoperative duplex ultrasound of the legs. Another screening method is the evaluation of D-dimer in a blood sample [16, 18, 19, 21, 30, 36, 41]. To further evaluate PE, computed tomography (CT) of the chest [3, 7, 18, 40, 42, 43] or angiography (CTA) [1, 6, 10, 20, 38] is the most commonly performed evaluation method; other evaluation methods are a ventilation–perfusion scan (VQ scan) [6, 39, 43] or a the pulmonary arteriogram [39].
Data extraction and analysis
Data was extracted from each article by one investigator (ZZ) and reviewed by a second investigator (CV-L). Disagreements between the reviewers were resolved by consensus. The following data were extracted from the included studies: study design, sample size, type of neurosurgical intervention (intracranial or spinal), type of antithrombotic prophylaxis, method of DVT diagnosis, and method of PE diagnosis, and, if mentioned, incidence of hemorrhage. The primary outcome parameter assessed was the occurrence of DVT and/or PE. If risk factors for VTE were evaluated, odds ratios or hazard ratios for each risk factor that was analyzed in a multivariate analysis were collected.
In order to calculate the average incidence of VTE in neurosurgical patients, data were pooled.
Results
Search and selection results
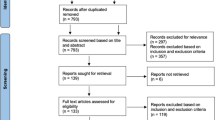
The search yielded 1969 unique references. After screening titles and abstracts, 82 articles were subjected to full text review (Fig. 1). A total of 51 studies were excluded after full text review due to the absence of specific information about the diagnostic method for DVT and only reporting bleeding complications. Twenty-five articles were subjected to quality assessment. Due to insufficient data, pooling of the data was not deemed meaningful, and only a descriptive analysis was performed.
Study characteristics
Of the 25 included studies, 1 was a randomized controlled trial [35], 3 were comparative studies [8, 13, 14], and 21 were case series (Tables 1 and 2). Twelve studies reported patients subjected to intracranial surgery. Two studies reported patients undergoing spinal surgery. Eleven studies reported patients both subjected to intracranial and spinal surgery (intracranial/spinal). Eight studies diagnosed DVT on clinical grounds [7, 10, 13, 14, 38, 41,42,43], and sixteen studies diagnosed DVT based on systematic screening methods, using D-dimer [16, 18, 19, 21, 30, 36], DUS [1,2,3, 6, 8, 16, 18,19,20,21, 25, 30, 34,35,36, 47], or CT [47]. One study evaluated both on clinical grounds and performed routine screening (DUS/CT), with the purpose of comparing the two methods [40]. Low molecular weight heparin (LMWH), unfractionated heparin, intermittent pneumatic compression (IPC) devices, compression stockings (CS) peri- and/or postoperatively, or a combination of these were described as prophylaxis methods (Table 3).
Assessment of risk of bias
Thirteen out of the 21 case series were rated to have a low risk of bias, having a score of 5/9 points on the Newcastle–Ottawa Quality Assessment Scale (Table 1), and one of the comparative studies (the RCT) was assessed to have a low risk of bias. The other three comparative studies were all scored to have a high risk of bias (Table 2).
VTE incidence related to prophylaxis strategy in intracranial surgery
The incidence of VTE in patients after intracranial surgery ranged from 1.3 to 26.4% (Table 3). Only one study yielded data for absence of prophylaxis for VTE and reported an incidence of 4.0%; diagnosis was made on clinical grounds without systematic screening of all patients. In patients receiving pharmacological prophylaxis (LMWH or heparin), the incidence of VTE diagnosis ranged from 1.3 to 7.9% (using systematic screening methods) and 2.0 to 3.8% if diagnosis was based on clinical evaluation. A deviating outcome was reported by Smith [43], evaluating VTE on clinical grounds and demonstrating an incidence of 15.8%. Only one article studied the incidence of DVT in patients being subjected to IPC as single prevention method and reported an incidence of 10.2% (screening by DUS). In the two articles describing patients who received both IPC and CS, the incidence was 13.4% evaluated on clinical grounds and 21.3% if DVT was diagnosed based on systematic screening. In patients in whom the administration of pharmacologic prophylaxis was combined with CS and/or IPC, the VTE rate was ranging from 3.1 to 6.4% based on clinical evaluation and from 7.3 to 26.4% based on systematic screening methods.
VTE incidence related to prophylaxis strategy in spinal surgery
One article studied the incidence of VTE using both IPC and CS as prophylactic method and reported a 29.4% incidence of VTE using DUS as systematic screening method. In patients in whom the administration of pharmacologic prophylaxis was combined with CS and/or IPC, the VTE rate was 0.6% based on systematic screening methods.
VTE incidence related to prophylaxis strategy in intracranial/spinal surgery
The incidence of VTE in patients after intracranial/spinal surgery ranged from 0.6 to 31.1% (Table 3). If no prophylaxis for VTE was provided, incidence was reported to be 10.3%, using systematic screening. In patients receiving pharmacologic prophylaxis such as LMWH and heparin, a 31.1% incidence of VTE was reported using systematic screening. In patients in whom the administration of pharmacologic prophylaxis combined with CS and/or IPC, the VTE rate was 7.1 to 12.7% based on clinical evaluation and 0.6 to 30.9% based on systematic screening methods.
VTE incidence categorized by prophylaxis strategy
VTE incidence was categorized by prophylaxis strategy in Table 4. If no prophylaxis for VTE was provided, VTE was reported to occur in 4.0 (clinical evaluation) to 10.3% (systemic evaluation) of patients. In patients receiving pharmacologic prophylaxis such as LMWH and heparin, a 1.3 to 31.1% incidence of VTE was reported. In patients who received IPC with CS, the incidence was 13.4 to 29.4%, while the incident in patients who received IPC alone was 10.2%. In patients in whom the administration of pharmacologic prophylaxis combined with IPC and/or CS, the VTE rate was 0.6 to 22.3%, and in patients whom the administration of pharmacologic prophylaxis combined with CS (without IPC), the VTE rate was 0.6 to 29.8%.
Pulmonary embolism in neurosurgical patients
Seventeen of the 31 studies reported the incidence of PE in neurosurgical patients (Table 3) [1, 3, 6,7,8,9,10, 12,13,14, 18, 20, 24, 33, 34, 38, 41].
The reported incidence of PE varied from 0 to 7.9%. A PE incidence of 0 to 4.5% was reported after intracranial surgery, 0.4 to 2.8% after spinal surgery, and 0.2 to 7.9% after intracranial/spinal surgery. The limited amount of data on the incidence of PE after neurosurgical procedures without antithrombotic prophylaxis reported an incidence of 1.0% after intracranial surgery.
Studies that provided pharmacologic prophylaxis report a PE incidence of 0 to 4.5% after intracranial surgery and 0.4% after intracranial/spinal surgery, that of mechanical prophylaxis a PE incidence of 0.5 to 3.4% after intracranial surgery, and that of both pharmacologic and mechanical prophylaxis a PE incidence of 0.3 to 2.6% after intracranial surgery and 0.2 to 7.9% after intracranial/spinal surgery.
Best evidence synthesis
Our analysis yielded 14 studies with a low risk of bias (Tables 1 and 2, printed in bold; Table 5) [1, 2, 6, 10, 16, 19,20,21, 25, 30, 34, 35, 40, 41]. In these studies, the reported incidence of VTE was 4.0% (clinical evaluation) in patients not receiving antithrombotic prophylaxis [10]. In patients receiving pharmacological prophylaxis in intracranial/spinal surgery, VTE rate was 31.1% [16] using systematic screening methods and 2.0% [10] using clinical evaluation. In patients who received mechanical prophylaxis, the incidence was 12.9% [41] evaluated on clinical grounds and systematic screening yielded 10.2% [20] or 21.3% [30] in intracranial surgery and 29.4% [19] in spinal surgery. In patients in whom the administration of pharmacological prophylaxis was combined with mechanical prophylaxis, systematic screening yielded VTE rates of 7.3% and 26.4% [35] in intracranial surgery (0.6 [1] to 30.9% [25] in intracranial/spinal surgery), while mere clinical evaluation yielded a percentage of 10.6 [40].
In these studies, the reported incidence of PE varied from 0 [10] to 3.4% [41]. The incidence of PE after neurosurgical procedures without antithrombotic prophylaxes reported an incidence of 1.0% [10]. Studies that provided pharmacologic prophylaxis report a PE incidence of 0% [10] and 0.4% [1], providing mechanical prophylaxis a PE incidence of 0.5 [41] to 3.4% [20] and providing both pharmacologic and mechanical prophylaxis a PE incidence of 0.6% [2], 0.3% [34], and 2.9% [6].
Due to insufficient data, pooling of the data was not deemed meaningful, and only a descriptive analysis was performed.
Risk factors
Data on risk factors associated with VTE revealed many independent risk factors associated with increased odds of VTE: older age [6, 7, 16, 21, 25, 38], presence of pre- or post-op motor deficit [6, 7, 16, 20, 25, 38], lower Karnofsky Performance Scale score [6, 7], peri-operation treatment with dehydration drugs and fibrin-based sealants [16, 30], increase in postoperative days in intensive care [38], intubated > 24 h/reintubated [38], history of VTE [38], presence of tumor [16], tumor histology [7], hypertension [7, 16, 25], infection [30], DM [20], and increased D-dimers [21, 25] (Table 6). The occurrence of VTE was associated with a longer hospitalization period [21, 25]. Spine surgery is associated with decreased odds of VTE [21]. Gender as a risk factor displayed contradictory results [21, 25].
Hemorrhage incidence
Data on the incidence of postoperative hemorrhage can possibly related to LMWH as prophylactic treatment for TE and is therefore relevant with respect to the topic [1,2,3, 6, 8, 10, 14, 30, 38, 42, 43]. The reported incidence of postoperative hemorrhage ranged from 0 to 9.1% (Table 7). Studies that provided pharmacological prophylaxis report a postoperative hemorrhage incidence varying from 0 to 9.1%, and those providing both pharmacological and mechanical prophylaxis reported a postoperative hemorrhage incidence varying from 0.6 to 6.7%. Only one of the articles providing only mechanical prophylaxis provides data on postoperative hemorrhage and reports an incidence of 1.6% [30].
Discussion
The prophylaxis strategies to prevent VTE in neurosurgery vary widely [14, 40, 42]. Moreover, the reported incidence of thromboembolic complications with the applied strategies covers a wide range as well [13, 16, 25, 26, 34, 38]. VTE incidence was reported to be substantially higher if the evaluation was done by a systematic screening method in comparison to a clinical evaluation method [35]. Results on incidence of VTE were grouped by type of operation (longer duration and longer anticipated immobilization), type of prophylaxis, and alleged risk of bias in the articles, in order to provide unequivocal results. We have to conclude though that the data available in literature do not allow drawing more specific conclusions on the effectiveness of prophylactic measures to lower the incidence of VTE than reported in our previous review.
It was assumed that interventions on the spine were less invasive than the intracranial procedures and that reported incidence of VTE would be lower in this group of patients. However, data were scarce to begin with and moreover varied widely. This is presumably due to the difference in nature of the spinal interventions. Even the two articles that reported data on specifically spinal surgery patients yielded very different data: one article reports a 0.6% incidence [2] and the other one a 29.4% incidence [19]. Presumably, the nature of the evaluated spinal interventions is different, but the articles do not elaborate on the specific interventions, making it impossible to draw valuable conclusions.
Detection of subclinical DVT
The question remains whether it is advisable to perform a postoperative evaluation of (subclinical) presence of DVT. The CHEST Guidelines, the evidence-based clinical practice guidelines concerning thromboembolic prevention developed by the American College of Chest Physicians, only recommends to perform a DUS screening in neurosurgical patients who are at high risk for VTE [15]. High risk for VTE is defined as the presence of a SCI or major head injury, without further specification. In our study, we demonstrated that DVT occurs more often without clinical symptoms (1.3 to 7.9% in patients who received pharmacological prophylaxis, 21.3% in IPC combined with CS, and 7.3 to 26.4% if pharmacologic prophylaxis was combined with CS and/or IPC in intracranial surgery and 0.6 to 30.9% if pharmacologic prophylaxis was combined with CS and/or IPC in intracranial/spinal surgery) than that DVT does lead to the conventional triad of symptoms in the leg (swelling, redness, and pain in the calf) [11, 17]. Subclinical DVT is associated with the formation of PE [29], which is a life-threatening complication. Therefore, systematic detection and prevention of subclinical DVT may be considered essential to prevent the serious complication of PE. In order to avoid performing a DUS for all neurosurgical patients in preparation of surgery, the Caprini Score for risk assessment of venous thromboembolism [5] could be used. The Caprini Score gives four risk groups (low, moderate, high, highest risk), and it can be useful to determine which patients should be preoperatively screened. Nonetheless, high-quality reports evaluating the true VTE incidence in neurosurgical patients are lacking, and thus, a trial to evaluate the subclinical incidence and possible risk factors for the presence of DVT is mandatory in order to decide which patients should be routinely screened postoperatively.
Risk factors for developing venous thromboembolism
Virchow’s triad classically explains the risk factors for VTE: stasis of blood, endothelial injury, and hypercoagulability [27]. Blood stasis is more likely in patients being subjected to long surgery duration and thus longer immobilization, with paresis/paralysis of the legs and with a poor Karnofsky Performance Scale (KPS) score, and are thus more likely to develop thromboembolic complications. The presence of a malignant tumor, especially higher-grade tumors [7], can interact with the host coagulation system and lead to a hypercoagulable state and thus cause VTE [4]. The risk factors that were evaluated in the articles in this review cover a very wide range and address all these factors. Ideally, these factors should be combined with a “thrombosis risk factor assessment” like the Caprini Score in order to optimize a choice for perioperative prophylactic therapy.
Postoperative Hemorrhage
Perioperative pharmacological anticoagulant therapy in order to prevent VTE theoretically increases the risk for postoperative hemorrhage. Consequently, the Chest Guideline does not recommend pharmacologic anticoagulant therapy for patients with high hemorrhage risk [15]. Only one of the articles describing the risk on postoperative hemorrhage concerns a prophylactic strategy without pharmacological anticoagulant therapy and gives a percentage of 1.6% [30]. This percentage is in the low range of the hemorrhage percentages demonstrated in the other 11 articles in which hemorrhage is described to range from 0 to 9.1% (Table 7) [1,2,3, 6, 8, 10, 14, 38, 42, 43]. However, the prevalence of hemorrhage is influenced by several other factors, which are not, or only scarcely, mentioned in the articles, and thus, no meaningful conclusions can be drawn.
Limitations and external validity
Limitations of this review are the heterogeneity of the type of surgical interventions with varying durations, of diagnostic methods, and of applied antithrombotic prophylaxis therapies. Even more importantly, the pathology of the patients, even within one study, varied. VTE incidence is known to be higher in patients with tumor or trauma, and in some studies, patient populations are mixed, while others are more specifically evaluating patients without trauma or tumor [6, 7, 10, 13, 16, 18, 20, 30, 38, 41,42,43]. Furthermore, two studies used different kinds of pharmacologic prophylaxis in one group [3, 43], and four studies did not describe the method of prophylaxis precisely [1, 25, 40, 47]. This inconsistency between articles induces selection bias, which makes it inadmissible to draw firm conclusions.
Clinical implementation
A trial to investigate effectiveness of different thromboembolic prophylactic strategies to prevent VTE should consider type and duration of the surgical intervention and take patient-related risk factors into account. In order to evenly distribute these properties over the groups to be evaluated, a randomized controlled trial setup is the most appropriate. Furthermore, it could be considered to start prophylactic strategy perioperative instead of postoperative, because of the long immobilization and hypercoagulability during the surgical intervention.
Conclusion
Incidence of VTE cannot be represented by only one percentage, but should be specified with respect to type of intervention, duration of immobilization, and presence of risk factors. Low molecular weight heparin, compression stockings, and intermittent pneumatic compression devices were all evaluated to give reduction in VTE, but with the currently available data, no conclusion can be drawn on generalizing the optimum treatment strategy to lower the incidence of thromboembolic complications. The data on incidence and risk factors however can contribute to optimizing prophylactic regimens in individual patients.
Data availability
Not applicable.
Code availability
Not applicable.
References
Agarwal N, Zenonos GA, Agarwal P, Walch FJ, Roach E, Stokes SJ, Friedlander RM, Gerszten PC (2019) Risk-to-benefit ratio of venous thromboembolism prophylaxis for neurosurgical procedures at a quaternary referral center. Neurosurgery 84:355–361. https://doi.org/10.1093/neuros/nyy035
Al-Dujaili TM, Majer CN, Madhoun TE, Kassis SZ, Saleh AA (2012) Deep venous thrombosis in spine surgery patients: incidence and hematoma formation. Int Surg 97(2):150–154. https://doi.org/10.9738/CC71.1
Ali NES, Alyono JC, Song Y, Kouhi A, Blevins NH (2021) Postoperative venous thromboembolism after neurotologic surgery. J Neurol Surg Part B: Skull Base 82(3):378–382. https://doi.org/10.1055/s-0039-3400223
Caine GJ, Stonelake PS, Lip GY, Kehoe ST (2002) The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 4:465–473. https://doi.org/10.1038/sj.neo.7900263
Caprini JA (2005) Thrombosis risk assessment as a guide to quality patient care. Dis Mon 51:70–78. https://doi.org/10.1016/j.disamonth.2005.02.003
Carrabba G, Riva M, Conte V, Di Cristofori A, Caroli M, Locatelli M, Castellani M, Bucciarelli P, Artoni A, Stocchetti N, Martinelli I, Rampini P (2018) Risk of post-operative venous thromboembolism in patients with meningioma. J Neurooncol 138:401–406. https://doi.org/10.1007/s11060-018-2810-z
Chaichana KL, Pendleton C, Jackson C, Martinez-Gutierrez JC, Diaz-Stransky A, Aguayo J, Olivi A, Weingart J, Gallia G, Lim M, Brem H, Quinones-Hinojosa A (2013) Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res 35:206–211. https://doi.org/10.1179/1743132812y.0000000126
Chibbaro S, Cebula H, Todeschi J, Fricia M, Vigouroux D, Abid H, Kourbanhoussen H, Pop R, Nannavecchia B, Gubian A, Prisco L, Ligarotti GKI, Proust F, Ganau M (2018) Evolution of prophylaxis protocols for venous thromboembolism in neurosurgery: results from a prospective comparative study on low-molecular-weight heparin, elastic stockings, and intermittent pneumatic compression devices. World Neurosurg 109:e510–e516. https://doi.org/10.1016/j.wneu.2017.10.012
Cloney MB, Goergen J, Hopkins BS, Dhillon ES, Dahdaleh NS (2018) Factors associated with venous thromboembolic events following ICU admission in patients undergoing spinal surgery: an analysis of 1269 consecutive patients. J Neurosurg Spine 30:99–105. https://doi.org/10.3171/2018.5.Spine171027
Daley MJ, Ali S, Brown CV (2015) Late venous thromboembolism prophylaxis after craniotomy in acute traumatic brain injury. Am Surg 81:207–211
Dermody M, Alessi-Chinetti J, Iafrati MD, Estes JM (2011) The utility of screening for deep venous thrombosis in asymptomatic, non-ambulatory neurosurgical patients. J Vasc Surg 53:1309–1315. https://doi.org/10.1016/j.jvs.2010.10.115
Dhillon ES, Khanna R, Cloney M, Roberts H, Cybulski GR, Koski TR, Smith ZA, Dahdaleh NS (2017) Timing and risks of chemoprophylaxis after spinal surgery: a single-center experience with 6869 consecutive patients. J Neurosurg Spine 27:681–693. https://doi.org/10.3171/2017.3.Spine161076
Ebeling M, Lüdemann W, Frisius J, Karst M, Schedel I, Gerganov V, Samii A, Fahlbusch R (2018) Venous thromboembolic complications with and without intermittent intraoperative and postoperative pneumatic compression in patients with glioblastoma multiforme using intraoperative magnetic resonance imaging. A retrospective study Neurochirurgie 64:161–165. https://doi.org/10.1016/j.neuchi.2018.04.007
Eisenring CV, Neidert MC, SabanésBové D, Held L, Sarnthein J, Krayenbühl N (2013) Reduction of thromboembolic events in meningioma surgery: a cohort study of 724 consecutive patients. PLoS One 8:e79170. https://doi.org/10.1371/journal.pone.0079170
Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW (2008) Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133:381s-453s. https://doi.org/10.1378/chest.08-0656
Guo F, Shashikiran T, Chen X, Yang L, Liu X, Song L (2015) Clinical features and risk factor analysis for lower extremity deep venous thrombosis in Chinese neurosurgical patients. J Neurosci Rural Pract 6:471–476. https://doi.org/10.4103/0976-3147.169801
Hamilton MG, Hull RD, Pineo GF (1994) Venous thromboembolism in neurosurgery and neurology patients: a review. Neurosurgery 34:280–296; discussion 296. https://doi.org/10.1227/00006123-199402000-00012
Hoefnagel D, Kwee LE, Van Putten EHP, Kros JM, Dirven CMF, Dammers R (2014) The incidence of postoperative thromboembolic complications following surgical resection of intracranial meningioma. A retrospective study of a large single center patient cohort. Clin Neurol Neurosurg 123:150–154. https://doi.org/10.1016/j.clineuro.2014.06.001
Ikeda T, Miyamoto H, Hashimoto K, Akagi M (2017) Predictable factors of deep venous thrombosis in patients undergoing spine surgery. J Orthop Sci 22:197–200. https://doi.org/10.1016/j.jos.2016.11.014
Kaewborisutsakul A, Tunthanathip T, Yuwakosol P, Inkate S, Pattharachayakul S (2020) Incidence and risk factors for venous thromboembolism following craniotomy for intracranial tumors: a cohort study. Asian J Neurosurg 15:31–38. https://doi.org/10.4103/ajns.AJNS_351_19
Karsy M, Azab MA, Harper J, Abou-Al-Shaar H, Guan J, Eli I, Brock AA, Ormond RD, Hosokawa PW, Gouripeddi R, Butcher R, Cole CD, Menacho ST, Couldwell WT (2020) Evaluation of a D-dimer protocol for detection of venous thromboembolism. World Neurosurgery 133:E774–E783. https://doi.org/10.1016/j.wneu.2019.09.160
Kearon C (2003) Natural history of venous thromboembolism. Circulation 107:I22-30. https://doi.org/10.1161/01.Cir.0000078464.82671.78
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR (2012) Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:e419S-e496S. https://doi.org/10.1378/chest.11-2301
Kimmell KT, Jahromi BS (2015) Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J Neurosurg 122:1004–1011. https://doi.org/10.3171/2014.10.Jns14632
Li J, Ren X, Zhu X, Chen H, Lin Z, Huang M, Gu Z (2020) Clinical predictive factors of lower extremity deep vein thrombosis in relative high-risk patients after neurosurgery: a retrospective study. Dis Markers 2020:5820749. https://doi.org/10.1155/2020/5820749
Lukassen J, Groen J, Jacobs W, Vleggeert-Lankamp C (2015) Thromboembolic prophylaxis in neurosurgical practice: a review. J Spine 4:2
Mammen EF (1992) Pathogenesis of venous thrombosis. Chest 102:640s–644s. https://doi.org/10.1378/chest.102.6_supplement.640s
Masuda EM, Kistner RL (2010) The case for managing calf vein thrombi with duplex surveillance and selective anticoagulation. Dis Mon 56:601–613. https://doi.org/10.1016/j.disamonth.2010.06.011
Moser KM, LeMoine JR (1981) Is embolic risk conditioned by location of deep venous thrombosis? Ann Intern Med 94:439–444. https://doi.org/10.7326/0003-4819-94-4-439
Nakano F, Matsubara T, Ishigaki T, Hatazaki S, Mouri G, Nakatsuka Y, Suzuki H (2018) Incidence and risk factor of deep venous thrombosis in patients undergoing craniotomy for brain tumors: a Japanese single-center, retrospective study. Thromb Res 165:95–100. https://doi.org/10.1016/j.thromres.2018.03.016
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372:n71. https://doi.org/10.1136/bmj.n71
Palareti G, Cosmi B, Lessiani G, Rodorigo G, Guazzaloca G, Brusi C, Valdré L, Conti E, Sartori M, Legnani C (2010) Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: the blind, prospective CALTHRO study. Thromb Haemost 104:1063–1070. https://doi.org/10.1160/th10-06-0351
Park JH, Lee KE, Yu YM, Park YH, Choi SA (2019) Incidence and risk factors for venous thromboembolism after spine surgery in Korean patients. World Neurosurg 128:e289–e307. https://doi.org/10.1016/j.wneu.2019.04.140
Patel AP, Koltz MT, Sansur CA, Gulati M, Hamilton DK (2013) An analysis of deep vein thrombosis in 1277 consecutive neurosurgical patients undergoing routine weekly ultrasonography. J Neurosurg 118:505–509. https://doi.org/10.3171/2012.11.Jns121243
Prell J, Schenk G, Taute BM, Scheller C, Marquart C, Strauss C, Rampp S (2018) Reduced risk of venous thromboembolism with the use of intermittent pneumatic compression after craniotomy: a randomized controlled prospective study. J Neurosurg:1–7. https://doi.org/10.3171/2017.9.Jns17533
Rethinasamy R, Alias A, Kandasamy R, Raffiq A, Looi MC, Hillda T (2019) Deep vein thrombosis and the neurosurgical patient. Malaysian J Med Sci 26(5):139–147. https://doi.org/10.21315/mjms2019.26.5.13
Righini M, Paris S, Le Gal G, Laroche JP, Perrier A, Bounameaux H (2006) Clinical relevance of distal deep vein thrombosis. Rev Lit Data Thromb Haemost 95:56–64
Rinaldo L, Brown DA, Bhargav AG, Rusheen AE, Naylor RM, Gilder HE, Monie DD, Youssef SJ, Parney IF (2019) Venous thromboembolic events in patients undergoing craniotomy for tumor resection: incidence, predictors, and review of literature. J Neurosurg 132:10–21. https://doi.org/10.3171/2018.7.Jns181175
Rolston JD, Han SJ, Bloch O, Parsa AT (2014) What clinical factors predict the incidence of deep venous thrombosis and pulmonary embolism in neurosurgical patients? J Neurosurg 121:908–918. https://doi.org/10.3171/2014.6.Jns131419
Samuel S, Patel N, McGuire MF, Salazar M, Nguyen T (2019) Analysis of venous thromboembolism in neurosurgical patients undergoing standard versus routine ultrasonography. J Thromb Thrombolysis 47:209–215. https://doi.org/10.1007/s11239-018-1761-8
Shi S, Cheng J, Chen H, Zhang Y, Zhao Y, Wang B (2020) Preoperative and intraoperative predictors of deep venous thrombosis in adult patients undergoing craniotomy for brain tumors: a Chinese single-center, retrospective study. Thromb Res 196:245–250. https://doi.org/10.1016/j.thromres.2020.09.005
Sjåvik K, Bartek J Jr, Solheim O, Ingebrigtsen T, Gulati S, Sagberg LM, Förander P, Jakola AS (2016) Venous thromboembolism prophylaxis in meningioma surgery: a population-based comparative effectiveness study of routine mechanical prophylaxis with or without preoperative low-molecular-weight heparin. World Neurosurg 88:320–326. https://doi.org/10.1016/j.wneu.2015.12.077
Smith TR, Lall RR, Graham RB, McClendon J Jr, Lall RR, Nanney AD, Adel JG, Zakarija A, Chandler JP (2014) Venous thromboembolism in high grade glioma among surgical patients: results from a single center over a 10 year period. J Neurooncol 120:347–352. https://doi.org/10.1007/s11060-014-1557-4
Stecker M, Michel K, Antaky K, Cherian S, Koyfmann F (2014) Risk factors for DVT/PE in patients with stroke and intracranial hemorrhage. Open Neurol J 8:1–6. https://doi.org/10.2174/1874205x01408010001
Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, Lake E, Murin S, Vintch JRE, Wells PS, Moores LK (2021) Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest 160:e545–e608. https://doi.org/10.1016/j.chest.2021.07.055
Wells G SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Yun R, Sciubba DM, Lewin JJ 3rd, Streiff MB, Haut ER, Lau BD, Shermock KM, Kraus PS, Popoola VO, Dalpoas SE (2020) Defects in processes of care for pharmacologic prophylaxis are common among neurosurgery patients who develop in-hospital postoperative venous thromboembolism. World Neurosurg 134:e664–e671. https://doi.org/10.1016/j.wneu.2019.10.163
Acknowledgements
We appreciate Jan Schoone for developing search strategy and performing literature search.
Funding
This article was funded by Chinese Scholarship Council (202207720054).
Author information
Authors and Affiliations
Contributions
Zhaoyuan Zhang and Carmen Vleggeert-Lankamp selected articles independently. Zhaoyuan Zhang, Husule Cai, and Carmen Vleggeert-Lankamp independently performed a risk of bias. Zhaoyuan Zhang and Carmen Vleggeert-Lankamp collected the data and performed the analysis. Zhaoyuan Zhang wrote the paper. Carmen Vleggeert-Lankamp helps to revise the paper.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Cai, H. & Vleggeert-Lankamp, C.L.A. Thromboembolic prophylaxis in neurosurgical practice: a systematic review. Acta Neurochir 165, 3119–3135 (2023). https://doi.org/10.1007/s00701-023-05792-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05792-3