Abstract
Background
Although there is an increasing body of evidence showing gender differences in various medical domains as well as presentation and biology of pituitary adenoma (PA), gender differences regarding outcome of patients who underwent transsphenoidal resection of PA are poorly understood. The aim of this study was to identify gender differences in PA surgery.
Methods
The PubMed/MEDLINE database was searched up to April 2023 to identify eligible articles. Quality appraisal and extraction were performed in duplicate.
Results
A total of 40 studies including 4989 patients were included in this systematic review and meta-analysis. Our analysis showed odds ratio of postoperative biochemical remission in males vs. females of 0.83 (95% CI 0.59–1.15, P = 0.26), odds ratio of gross total resection in male vs. female patients of 0.68 (95% CI 0.34–1.39, P = 0.30), odds ratio of postoperative diabetes insipidus in male vs. female patients of 0.40 (95% CI 0.26–0.64, P < 0.0001), and a mean difference of preoperative level of prolactin in male vs. female patients of 11.62 (95% CI − 119.04–142.27, P = 0.86).
Conclusions
There was a significantly higher rate of postoperative DI in female patients after endoscopic or microscopic transsphenoidal PA surgery, and although there was some data in isolated studies suggesting influence of gender on postoperative biochemical remission, rate of GTR, and preoperative prolactin levels, these findings could not be confirmed in this meta-analysis and demonstrated no statistically significant effect. Further research is needed and future studies concerning PA surgery should report their data by gender or sexual hormones and ideally further assess their impact on PA surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenoma (PA) is the second most frequent intracranial neoplasm and presents clinically as an incidental finding or with endocrine or mass effect manifestations [4]. Over the past decades, transsphenoidal surgery has established itself as the gold standard, first-line treatment for most subtypes of PA [15, 45, 82].
Recently, the influence of gender on clinical outcomes has seen a massive increase in interest among the scientific community, as outcome differences with clinical relevance have been established in various domains such as cardiovascular disease, autoimmune disease, and infectious disease. [11, 20, 41, 49, 50, 56, 62, 63, 65, 70, 80, 85, 87, 90]
Although there is literature concerning gender differences in the biology of the pituitary gland and in the presentation as well as the biology of PA, gender differences regarding the outcome of patients who underwent transsphenoidal resection of PA are poorly understood. [3, 25, 61, 63, 74, 76, 77, 88] It is currently unknown whether the different physiological hormone levels or any other gender differences may impact patient selection, success of treatment, or hormonal cut-off values [28, 32, 33].
Published data on this topic are scarce, and the authors are not aware of any literature review on gender differences in pituitary surgery, although there is one study assessing gender differences in non-surgical aspects of non-functioning PA (NFPA) [25]. Systematic reviews and meta-analyses can lead to more realistic results with better generalisability and less risk of bias compared to single studies [64]. In this study, we systematically reviewed the literature to evaluate the influence of gender and sexual hormones on outcomes after endoscopic or microscopic transsphenoidal PA surgery.
Materials and methods
Overview
A systematic review was carried out to identify any studies reporting at least one of (1) GTR (rate of radiological gross total resection), (2) rate of new endocrinological deficits, or (3) biochemical remission (for patients with hormone-secreting adenomas) after resection of PA stratified by gender or by preoperative sexual hormone (estrogen, testosterone, prolactin). Title and abstract screening, full-text review, and data extraction were handled independently by two reviewers (ST and SH), and disagreements at any stage were resolved by discussion and consensus. Persisting disagreements were resolved by discussion with a third reviewer (VS). We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol [64].
Search strategy
The PubMed/MEDLINE database was searched to identify eligible articles. The search strategy included combinations of the following terms: pituitary; adenoma; surgery; resection; transsphenoidal; gender; sex; male; female; prolactin; testosterone; estrogen; gross total resection; GTR; deficit; endocrine; endocrinological; and biochemical (Supplementary Table 2). Word variations and exploded medical subject headings were searched for whenever feasible. Additionally, reference lists were hand-searched to identify further studies of interest. The last comprehensive search was conducted on April 30, 2023.
Study selection
Only in vivo studies enrolling humans of all age groups in English, Italian, French, Dutch, and German were considered. As no controlled trials were anticipated, prospective and retrospective single-arm cohort studies and case series of adult individuals were also included. We excluded pediatric cases series. Case reports and small case series with less than 5 patients were excluded. To be considered, patients had to undergo endoscopic or microscopic transsphenoidal resection of PA. Studies had to assess at least one of the three abovementioned outcomes of interest stratified either by gender or by sexual hormone levels. In this way, we were able to rate the potential influence of sexual hormones and gender on outcomes. Studies reporting only resection of Rathke cleft cysts, craniopharyngiomas, or other lesions were excluded. We also excluded studies dealing mainly with transcranial or combined procedures. Studies reporting the outcomes of interest with a mix of targeted GTR and subtotal resection (STR) (i.e., a realistic caseload) were included. Exact cohort duplicates were excluded, although we did include updates of previously published cohorts with a sample size increase of at least 50%. Studies published before the 1st of January 1990 were excluded.
Data extraction and quality assessment
We extracted the following information if available from all included publications: study design and year of publication, number of patients, mean patient age and gender distribution, data on prolactin, testosterone, and estrogen levels, as well as data on GTR, new endocrinological deficits, and biochemical remission among patients with secreting adenomas. The methodological quality of included studies was graded using the GRADE framework [38].
Statistical meta-analysis
Based on anticipated heterogeneity and low event rates among studies, a random-effects analysis model (Mantel–Haenszel) that assesses odds ratios (OR) was chosen as the primary statistical method [39]. Cochran’s Q and I2 were used to evaluate heterogeneity, and a P < 0.1 was considered as relevant heterogeneity. All statistical analyses were carried out in RevMan version 5.4. Forest plots were generated to illustrate the main results of the meta-analysis.
Results
Literature search
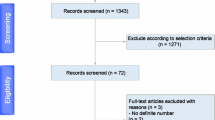
As seen in the PRISMA flowchart in Fig. 1, the PubMed/Medline search provided 3238 articles to which none were added through other sources. After duplicate removal (n = 35), 3203 records were screened and 294 were assessed for eligibility through full-text screening, concluding 40 studies included in qualitative synthesis, all of which were also eligible for quantitative meta-analysis [2, 6,7,8,9,10, 12, 14, 17, 21, 27, 29, 31, 34, 40, 42, 47, 48, 51,52,53, 55, 57,58,59, 67, 68, 75, 78, 79, 81, 83, 86, 89, 91,92,93,94,95,96].
Study characteristics
The details of the 40 included studies are summarized in Table 1. We identified 31 studies reporting postoperative biochemical remission [2, 6, 8, 10, 12, 14, 21, 29, 31, 34, 40, 42, 47, 48, 51,52,53, 55, 57,58,59, 67, 68, 75, 78, 79, 81, 86, 89, 92, 94], eight reporting rate of GTR [7, 17, 27, 68, 75, 91, 94, 95], five reporting incidence of postoperative diabetes insipidus (DI) [7, 21, 58, 83, 93], four reporting preoperative levels of prolactin [21, 67, 94, 96], one study reporting rate of postoperative hyperprolactinemia [94], one study reporting both postoperative adrenocorticotropic hormone (ACTH) and thyroid-stimulating hormone (TSH) deficiency [9], one study reporting postoperative panhypopituitarism [83], and one study reporting any endocrinological deficit [55], while each of those endpoints was stratified by gender. No studies were found that reported preoperative levels of testosterone or estrogen, postoperative follicle-stimulating hormone (FSH), luteinizing hormone (LH) deficiency, growth hormone (GH) deficiency, or postoperative rate of syndrome of inappropriate antidiuretic hormone secretion (SIADH) by gender. Endpoints reported by one or less study could not be analyzed and are reported in Supplementary Table 1.
Detailed qualitative interpretation of all analyzed outcomes including detailed certainty assessments is shown in Table 2.
Biochemical remission
Overall, 31 studies including 3605 patients (1410 male, 2195 female) were analyzed via random-effects meta-analysis, which showed an odds ratio of postoperative biochemical remission in males vs. females of 0.83 (95% CI 0.59–1.15). Heterogeneity was high with a I2-value of 71% (P < 0.00001) and the overall effect was 1.13 (P = 0.26) (Fig. 2). Twenty-three of the included studies were rated at a GRADE certainty of “very low” due to their small sample size while six were rated at a GRADE certainty of “low” (Table 1). Overall, after a detailed certainty assessment, the certainty of the outcome of this analysis was very low (Table 2).
Rate of GTR
In the evaluation of eight studies including 1155 patients (518 male, 637 female) via random-effects meta-analysis, an odds ratio of GTR in male vs. female patients showed to be 0.68 (95% CI 0.34–1.39). Again, heterogeneity was high with a I2-value of 74% (P = 0.0003) and the overall effect was 1.04 (P = 0.30) (Fig. 2). Five of the included studies were rated at a GRADE certainty of “very low” due to their small sample size while three were rated at a GRADE certainty of “low” (Table 1). Overall, after a detailed certainty assessment, the certainty of the outcome of this analysis was very low (Table 2).
Postoperative diabetes insipidus
The rate of postoperative DI in male vs. female patients was analyzed via random-effects meta-analysis including five studies with 598 patients (240 male, 358 female). The odds ratio was shown to be 0.40 (95% CI 0.26–0.64) with a low heterogeneity with a I2-value of 0% (P = 0.96). The overall effect was 3.92 (P < 0.0001) (Fig. 2). Three of the included studies were rated at a GRADE certainty of “very low” due to their small sample size while two were rated at a GRADE certainty of “low” (Table 1). Overall, after a detailed certainty assessment, the certainty of the outcome of this analysis was low (Table 2).
Preoperative level of prolactin
Four studies including 315 patients (108 male, 207 female) were analyzed via random-effects meta-analysis. The mean difference in the preoperative level of prolactin in male vs female patients was shown to be 11.62 (95% CI − 119.04–142.27) with high heterogeneity of I2 = 79% (P = 0.003). The overall effect was shown to be 0.17 (P = 0.86) (Fig. 2). Three of the included studies were rated at a GRADE certainty of “very low” due to their small sample size while one was rated at a GRADE certainty of “low” (Table 1). Overall, after a detailed certainty assessment, the certainty of the outcome of this analysis was very low (Table 2).
Discussion
The aim of this study was to identify gender differences in PA surgery. In our meta-analysis, there was a significantly higher rate of postoperative DI in female patients after endoscopic or microscopic transsphenoidal PA surgery, and although there was some data in isolated studies suggesting the influence of gender on postoperative biochemical remission, rate of GTR, and preoperative prolactin levels in patients after endoscopic or microscopic transsphenoidal PA surgery, these findings could not be confirmed in this meta-analysis and demonstrated no statistically significant effect of gender after endoscopic or microscopic PA surgery.
Gender differences are an important and established influence on clinical outcomes in various domains. [41, 50, 62, 63, 65, 70] While there is evidence of gender differences in clinical presentation and tumor size of PA [3, 16, 19, 25, 44, 68, 73, 84, 94], there is still little data available on its influence in PA surgery. Furthermore, in published studies, few data on baseline characteristics and outcomes concerning gender and sexual hormone status of participants is included: preoperative levels of testosterone or estrogen, postoperative FSH, LH or GH deficiency, or postoperative frequency of SIADH are not reported in any study and only single studies reported postoperative hyperprolactinemia [94], postoperative panhypopituitarism [83], postoperative ACTH and TSH deficiency [9], and any endocrinological deficit [55] stratified by gender (Supplementary Table 1).
In our analysis, we found overall no statistically significant impact of gender on biochemical remission of PA after surgery (Fig. 1). In single studies, however, differences by sex on biochemical remission are reported: Park et al. (2017) [68] found worse outcomes of male patients in comparison to premenopausal female patients with GH secreting adenomas. In addition, Yoo et al. [94] found worse outcomes in male than female patients with prolactinomas, and Arasho et al. [3] reported a worse outcome in male than female patients with prolactinoma, although in patients with non-functioning PA, significantly worse outcomes were observed in female than in male patients. The reasons for both these findings remain unclear but might be explained due to the difference in the distribution of patients with female patients with prolactinoma typically presenting at a lower age while not only older age at presentation but also larger tumor size and a possibly more aggressive biology of male patients with prolactinoma have been discussed, which in turn might lead to better outcomes in female patients [1, 13, 18, 19, 23, 24, 26, 94]. Although in prolactinoma a difference in the distribution of patients may be the reason for gender-specific outcomes, in other subtypes of PA the reason for such a difference, as in single studies there seems to be, remains unclear and future studies are needed to not only fully establish such a difference, but also a possible reason for it [3, 68]. The findings of these single studies suggest that in specific subtypes of PA and in specific age groups, sex and gender might have an impact on the biochemical remission of PA after surgery.
The impact of gender on GTR also showed no statistically significant difference between male and female patients. These findings are consistent with the findings of Park et al. (2017) [68], where no statistically significant influence of gender on the rate of GTR was found. Contrary to these findings, Yoo et al. (2018) [94] reported a significantly lower number of GTR in male patients, although this study included a much lower sample size than the analysis of this study and the study of Park et al. [68].
In our analysis, we found a significantly higher rate of postoperative DI in female than male patients. With a low heterogeneity of our analysis, these findings are consistent with included studies. It should be mentioned that there is one study by Joshi et al. [43] which specified postoperative DI by gender for all transsphenoidal surgery and, when analyzing for PA surgery alone, did not find a statistically significant difference of gender on postoperative DI and did not discuss this in detail. Although the rate of postoperative DI in female patients was significantly higher in our analysis, the possible reason for this outcome remains largely unclear. One possible explanation for this difference is a possible age difference between female and male patients at presentation, as prior studies have shown there to be a difference in age of presentation regarding gender in different subtypes of PA, most notably in prolactinoma, as mentioned above [63, 94]. In the studies included in this analysis, the two biggest studies Tiwari et al. [83] and Yasuda et al. [93] found lower age to be a risk factor for developing postoperative DI but neither of those studies analyzed or reported age of presentation by gender and its possible influence on this higher risk of postoperative DI at a lower age [83, 93]. So while there may be gender differences in age of presentation and both lower age at presentation and female gender have now been linked to higher rates of DI, it remains unclear if the two are linked or independent risk factors for postoperative DI. Additionally, the underlying reasons for a higher incidence of DI in both remain unclear while both a more aggressive surgical approach in younger patients and a smaller pituitary gland with a therefore higher vulnerability to resection in females have been discussed [83, 93]. While in our analysis we found a low heterogeneity of included studies, it must be stated that definitions of DI did differ significantly between included studies, most noticeably in the two biggest included studies Tiwari et al. [83] and Yasuda et al. [93] While in Yasuda et al. [93] DI was defined as “(1) polyuria: urinary flow greater than 250 ml/h for more than 2 h and (2) urinary hypoosmolarity: defined as a urinary density less than 1005,” Tiwari et al. [83] simply defined DI as a prescription for desmopressin at the time of discharge. While considering that patients were operatively treated and hospitalized for PA adenoma and it is therefore highly likely that a prescription of desmopressin in this circumstance will have meant postoperative DI, we cannot be sure that in Tiwari et al. [83] the diagnostic criteria of DI was homogeneous in their institution over included years, that this DI is a new postoperative phenomenon, or that, in fact, desmopressin was prescriped for DI in the first place [22, 30, 54, 60, 71, 72].
The levels of preoperative prolactin did not differ significantly between male and female patients, although this part of the meta-analysis has to be carefully interpreted due to the high heterogeneity, low sample size, and due to the sensitivity of meta-analyses of mean differences toward non-normal distributions of the source data, which could not be judged from the original publications. Within the literature on medically treated prolactinomas, the studies of Delgrange et al. [24], Khare et al. [46], and Nishioka et al. [66] all reported a significantly higher level of pre-treatment prolactin in male than female patients and with that a strongly correlating tumor size. Reasons for these bigger tumors in male patients are controversial and might be explained by either a longer delay of diagnosis due to fewer early symptoms of hyperprolactinemia or the greater proliferation potential of these tumors in male patients [24, 46, 66]. As explained above, the meta-analysis on prolactin levels that was possible from the included studies has to be carefully considered. In addition, while in most cases medical treatment is the appropriate initial treatment for prolactinoma [5, 66, 69] and in the abovementioned studies all patients were at least initially treated medically, our analysis only included patients that underwent transsphenoidal PA surgery, and therefore, levels of serum prolactin might be different than in patients that are initially or purely treated medically.
In nearly all the analyzed endpoints, except for the rate of DI, there was high heterogeneity of included studies. This is most likely due to the very different results of included studies regarding the impact of gender on analyzed outcomes, which in turn might be due to the small to very small sample sizes of most included studies. While high heterogeneity is not particularly desired, it could not be avoided in our analysis due to the very little available literature on the influence of gender on PA surgery.
Most included studies (32/40) were given a GRADE rating of “very low” with the remaining studies rated “low” (Table 1). This rating again was due to the low to very low number of either patients overall or outcomes reported by gender in included studies.
In terms of certainty assessment, the risk of bias was rated as not serious in all analyzed outcomes according to the GRADE framework [37]. Inconsistency was rated serious in analyzed outcomes of biochemical remission and GTR. In both outcomes, this was due to a high heterogeneity and low sample size of included studies as mentioned above. In postoperative DI, inconsistency was rated as not serious, as the analysis showed a low heterogeneity. In preoperative levels of prolactin, inconsistency showed to be very serious, due to the abovementioned high heterogeneity, low sample size, and sensitivity of meta-analyses of mean difference toward non-normal distributions of data. Indirectness was rated as not serious in all analyzed outcomes according to the GRADE framework [35]. Imprecision was rated as serious in both postoperative biochemical remission and DI, as very serious in postoperative GTR, and as extremely serious in preoperative levels of prolactin according to the GRADE framework [36], while in all outcomes, plausible residual confounding like age, comorbidities, and surgical indication would reduce the demonstrated effect. Overall, according to the GRADE framework, the analysis of postoperative DI had a low overall certainty, while the analysis of postoperative biochemical remission, GTR, and preoperative levels of prolactin all had a very low overall certainty (Table 2). This overall level of certainty is not surprising considering there were relatively few studies reporting outcomes by gender, a low to very low sample size of those who did, and a therefore relatively low sample size in all analyzed outcomes with most, except for postoperative DI, showing high heterogeneity.
Although our meta-analysis did not find a statistically significant difference of gender in postoperative biochemical remission, rate of GTR, or preoperative prolactin levels, these findings do not establish that there is no difference of gender in these outcomes at all. As this study searched for differences in all transsphenoidal pituitary adenoma surgery regardless of specific subtype, this generalization can lead to misleading findings and possible influences of gender in these subtypes may be overlooked. Furthermore, we cannot assure the homogeneity of included studies concerning age, comorbidities, and surgical indication. Nonetheless, our analysis found a higher rate of postoperative DI in female than male patients and while a possible influence of gender on postoperative biochemical remission, rate of GTR, or preoperative prolactin could not be found in our meta-analysis, still, in single studies, it appears that gender may have an influence on outcomes after pituitary surgery.
While there was a significantly higher rate of postoperative DI in female patients after endoscopic or microscopic transsphenoidal PA surgery, our analysis of the influence of gender on postoperative biochemical remission, rate of GTR, and preoperative prolactin levels did not demonstrate a statistically significant effect. Further research and studies with larger sample sizes and considering PA subtypes and different age groups (premenopausal vs. postmenopausal) are needed to establish a clear understanding of their impact on PA surgery. While reporting data stratified by gender and sexual hormones would be relatively easy, there is still little data reported as such and its significance remains to be examined. Future studies concerning PA surgery should report their data by gender or sexual hormones and ideally further assess their impact on PA surgery.
Limitations
The main limitation of this study is the general lack of gender-specific data reported in publications and the therefore relatively small sample size, although reporting data by gender would be simple and might lead to new evidence regarding gender sciences and its impact on neurosurgery and medicine as a whole.
Furthermore, we analyzed all PA treated with endoscopic or microscopic transsphenoidal surgery as a group and, because of the limited reporting and small sample sizes, did not specify PA by subtype. Gender and sexual hormones might have varying influences on surgical outcomes of different subgroups and a generalization of all PA may lead to misleading results. Additionally, the homogeneity of included studies concerning age, comorbidities, and surgical indication cannot be assured.
Another limitation of this analysis is that most included studies classified PA according to clinical phenotype. While as mentioned our analysis did not differ between subtypes of PA, there might have been differences in the classification of PA in included studies as PA subtypes defined as its clinical phenotype is not always identical to respective pathological studies, and the classification of tumors may have changed over the time period in which included studies were published.
Moreover, we cannot be sure that included studies homogenously defined reported outcomes as, for example, and as mentioned before, postoperative DI was not homogenously defined in all included studies.
Additionally, due to the small sample sizes, there is high heterogeneity in our analyses, and as a meta-analysis, there might be inherent publication bias in this study.
Conclusions
After an extensive literature search, we analyzed 40 studies regarding the influence of gender on endoscopic or microscopic transsphenoidal PA surgery. In our meta-analysis, there was a significantly higher rate of postoperative DI in female patients after endoscopic or microscopic transsphenoidal PA surgery, and although there was some data in isolated studies suggesting the influence of gender on postoperative biochemical remission, rate of GTR, and preoperative prolactin levels, these findings could not be confirmed in this meta-analysis and demonstrated no statistically significant effect. Further research is needed and future studies concerning PA surgery should report their data by gender or sexual hormones and ideally further assess their impact on PA surgery.
Data Availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Abbreviations
- PA:
-
Pituitary adenoma
- NFPA:
-
Non-functioning pituitary adenoma
- GTR:
-
Gross total resection
- STR:
-
Subtotal resection
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluations
- OR:
-
Odds ratio
- DI:
-
Diabetes insipidus
- ACTH:
-
Adrenocorticotropic hormone
- TSH:
-
Thyroid-stimulating hormone
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- GH:
-
Growth hormone
- SIADH:
-
Syndrome of inappropriate antidiuretic hormone secretion
References
Akin S, Isikay I, Soylemezoglu F, Yucel T, Gurlek A, Berker M (2016) Reasons and results of endoscopic surgery for prolactinomas: 142 surgical cases. Acta Neurochir (Wien) 158(5):933–942
Antunes X, Ventura N, Camilo GB et al (2018) Predictors of surgical outcome and early criteria of remission in acromegaly. Endocrine 60(3):415–422
Arasho BD, Schaller B, Sandu N, Zenebe G (2009) Gender-related differences in pituitary adenomas. Exp Clin Endocrinol Diabetes 117(10):567–572
Araujo-Castro M, Berrocal VR, Pascual-Corrales E (2020) Pituitary tumors: epidemiology and clinical presentation spectrum. Hormones 19(2):145–155
Asano S, Ueki K, Suzuki I, Kirino T (2001) Clinical features and medical treatment of male prolactinomas. Acta Neurochir (Wien) 143(5):465–470
Asha MJ, Takami H, Velasquez C, Oswari S, Almeida JP, Zadeh G, Gentili F (2020) Long-term outcomes of transsphenoidal surgery for management of growth hormone–secreting adenomas: single-center results. J Neurosurg 133(5):1360–1370
Aydin S, Comunoglu N, Ahmedov ML, Korkmaz OP, Oz B, Kadioglu P, Gazioglu N, Tanriover N (2019) Clinicopathologic characteristics and surgical treatment of plurihormonal pituitary adenomas. World Neurosurg 130:e765–e774
Aydin S, Ozoner B, Sahin S, Alizada O, Comunoglu N, Oz B, Gazioglu N, Kadioglu P, Tanriover N (2020) A follow-up study on outcomes of endoscopic transsphenoidal approach for acromegaly. Clin Neurol Neurosurg 198:106201
Baumann F, Schmid C, Bernays R-L (2010) Intraoperative magnetic resonance imaging-guided transsphenoidal surgery for giant pituitary adenomas. Neurosurg Rev 33(1):83–90
Bellut D, Hlavica M, Schmid C, Bernays RL (2010) Intraoperative magnetic resonance imaging–assisted transsphenoidal pituitary surgery in patients with acromegaly. Neurosurg Focus 29(4):E9
Bögli SY, Utebay D, Smits N, Westphal LP, Hirsbrunner L, Unseld S, Keller E, Brandi G (2022) Sex-related differences of invasive therapy in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 164(11):2899–2908
Bora SK, Suri A, Khadgawat R et al (2020) Management of Cushing’s disease: changing trend from microscopic to endoscopic surgery. World Neurosurg 134:e46–e54
Burrow GN, Wortzman G, Rewcastle NB, Holgate RC, Kovacs K (1981) Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N Engl J Med 304(3):156–158
Campbell PG, Kenning E, Andrews DW, Yadla S, Rosen M, Evans JJ (2010) Outcomes after a purely endoscopic transsphenoidal resection of growth hormone–secreting pituitary adenomas. Neurosurg Focus 29(4):E5
Cappabianca P, Cavallo LM, de Divitiis E (2004) Endoscopic endonasal transsphenoidal surgery. Neurosurgery 55(4):933–940 (discussion 940-941)
Chen J, Schmidt RE, Dahiya S (2019) Pituitary adenoma in pediatric and adolescent populations. J Neuropathol Exp Neurol 78(7):626–632
Chen Y, Xu X, Cao J et al (2022) Transsphenoidal surgery of giant pituitary adenoma: results and experience of 239 cases in a single center. Front Endocrinol 13:879702
Ciccarelli A, Guerra E, De Rosa M, Milone F, Zarrilli S, Lombardi G, Colao A (2005) PRL secreting adenomas in male patients. Pituitary 8(1):39–42
Colao A, Sarno A, Cappabianca P, Briganti F, Pivonello R, Somma C, Faggiano A, Biondi B, Lombardi G (2003) Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocr 148(3):325–331
Corrao S, Santalucia P, Argano C et al (2014) Gender-differences in disease distribution and outcome in hospitalized elderly: data from the REPOSI study. Eur J Intern Med 25(7):617–623
Cote DJ, Smith TR, Sandler CN et al (2016) Functional gonadotroph adenomas. Neurosurgery 79(6):823–831
Cvetković RS, Plosker GL (2005) Desmopressin: in adults with nocturia. Drugs 65(1):99–107
Delgrange E, Sassolas G, Perrin G, Jan M, Trouillas J (2005) Clinical and histological correlations in prolactinomas, with special reference to bromocriptine resistance. Acta Neurochir (Wien) 147(7):751–758
Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J (1997) Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocr Metab 82(7):6
Di Somma C, Scarano E, de Alteriis G, Barrea L, Riccio E, Arianna R, Savastano S, Colao A (2021) Is there any gender difference in epidemiology, clinical presentation and co-morbidities of non-functioning pituitary adenomas? A prospective survey of a National Referral Center and review of the literature. J Endocrinol Invest 44(5):957–968
Fainstein Day P, Glerean M, Lovazzano S, Pietrani M, Christiansen S, Balzaretti M, Kozak A, Carrizo A (2010) Gender differences in macroprolactinomas: study of clinical features, outcome of patients and Ki-67 expression in tumor tissue. Front Horm Res 38:50–58. https://doi.org/10.1159/000318494. Epub 2010 Jul 5
Fallah N, Taghvaei M, Sadaghiani S, Sadrhosseini SM, Esfahanian F, Zeinalizadeh M (2019) Surgical outcome of endoscopic endonasal surgery of large and giant pituitary adenomas: an institutional experience from the Middle East. World Neurosurg 132:e802–e811
Fleseriu M, Biller BMK, Freda PU et al (2021) A Pituitary Society update to acromegaly management guidelines. Pituitary 24(1):1–13
Fomekong E, Maiter D, Grandin C, Raftopoulos C (2009) Outcome of transsphenoidal surgery for Cushing’s disease: a high remission rate in ACTH-secreting macroadenomas. Clin Neurol Neurosurg 111(5):442–449
Franchini M (2007) The use of desmopressin as a hemostatic agent: a concise review. Am J Hematol 82(8):731–735
Giese S, Nasi-Kordhishti I, Honegger J (2021) Outcomes of transsphenoidal microsurgery for prolactinomas – a contemporary series of 162 cases. Exp Clin Endocrinol Diabetes 129(03):163–171
Giustina A, Barkan A, Beckers A et al (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab 105(4):dgz096
Giustina A, Barkhoudarian G, Beckers A et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21(4):667–678
Guo X, Zhang R, Zhang D et al (2022) Determinants of immediate and long-term remission after initial transsphenoidal surgery for acromegaly and outcome patterns during follow-up: a longitudinal study on 659 patients. J Neurosurg 137(3):618–628
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 64(12):1303–1310
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 64(12):1283–1293
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y (2011) GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 64(4):407–415
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Higgins JPT, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from https://www.handbook.cochrane.org
Hofstetter CP, Mannaa RH, Mubita L, Anand VK, Kennedy JW, Dehdashti AR, Schwartz TH (2010) Endoscopic endonasal transsphenoidal surgery for growth hormone–secreting pituitary adenomas. Neurosurg Focus 29(4):E6
Huan C, Qu Y, Ren Z (2014) Gender differences in presentation and outcome of patients with Cushing’s disease in Han Chinese. Biomed Mater Eng 24(6):3439–3446
Jane JA, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, Marshall JC, Laws ER, Vance ML (2011) Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab 96(9):2732–2740
Joshi RS, Pereira MP, Osorio RC et al (2022) Identifying risk factors for postoperative diabetes insipidus in more than 2500 patients undergoing transsphenoidal surgery: a single-institution experience. J Neurosurg 137(3):647–657
Kane LA, Leinung MC, Scheithauer BW, Bergstralh EJ, Laws ER, Groover RV, Kovacs K, Horvath E, Zimmerman D (1994) Pituitary adenomas in childhood and adolescence. J Clin Endocrinol Metab 79(4):1135–1140
Kanter AS, Dumont AS, Asthagiri AR, Oskouian RJ, Jane JA, Laws ER (2005) The transsphenoidal approach. A historical perspective Neurosurg Focus 18(4):e6
Khare S, Lila AR, Patt H, Yerawar C, Goroshi M, Bandgar T, Shah NS (2016) Gender differences in macroprolactinomas: a single centre experience. Endocr Connect 5(1):20–27
Kim JH, Hur KY, Lee JH et al (2017) Outcome of endoscopic transsphenoidal surgery for acromegaly. World Neurosurg 104:272–278
Kim SH, Ku CR, Na M et al (2020) Immediate postoperative measurement of thyroid-stimulating hormone as an early predictor of remission in thyroid-stimulating hormone–secreting pituitary adenomas. J Neurosurg 134(3):794–800
Kitler ME (1992) Differences in men and women in coronary artery disease, systemic hypertension and their treatment. Am J Cardiol 70(11):1077–1080
Koenig J, Thayer JF (2016) Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev 64:288–310
Ku CR, Choe EY, Hong JW, Kim EH, Park SH, Kim SH, Lee EJ (2016) No differences in metabolic outcomes between nadir GH 0.4 and 1.0 ng/mL during OGTT in surgically cured acromegalic patients (observational study). Medicine (Baltimore) 95(24):e3808
Ku CR, Kim EH, Oh MC, Lee EJ, Kim SH (2012) Surgical and endocrinological outcomes in the treatment of growth hormone-secreting pituitary adenomas according to the shift of surgical paradigm. Oper Neurosurg 71:ons192–ons203
Lasolle H, Teulade M, Lapras V, Vasiljevic A, Borson-Chazot F, Jouanneau E, Raverot G (2022) Postoperative remission of non-invasive lactotroph pituitary tumor: a single-center experience. Ann Endocrinol 83(1):1–8
Leissinger C, Carcao M, Gill JC, Journeycake J, Singleton T, Valentino L (2014) Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 20(2):158–167
Little AS, Gardner PA, Fernandez-Miranda JC, Chicoine MR, Barkhoudarian G, Prevedello DM, Yuen KCJ, Kelly DF, __ (2019) Pituitary gland recovery following fully endoscopic transsphenoidal surgery for nonfunctioning pituitary adenoma: results of a prospective multicenter study. J Neurosurg 133(6):1732–1738
van Lunzen J, Altfeld M (2014) Sex differences in infectious diseases-common but neglected. J Infect Dis 209(suppl 3):S79–S80
Lyu L, Yin S, Hu Y, Chen C, Jiang Y, Yu Y, Ma W, Wang Z, Jiang S, Zhou P (2020) Hyperprolactinemia in clinical non-functional pituitary macroadenomas: a STROBE-compliant study. Medicine (Baltimore) 99(41):e22673
Mason RB, Oldfield EH (1997) Selective excision of adenomas originating in or extending into the pituitary stalk with preservation of pituitary function. J Neurosurg 87:9
Mayberg M, Reintjes S, Patel A, Moloney K, Mercado J, Carlson A, Scanlan J, Broyles F (2017) Dynamics of postoperative serum cortisol after transsphenoidal surgery for Cushing’s disease: implications for immediate reoperation and remission. J Neurosurg 129(5):1268–1277
McCarty TS, Shah AD (2023) Desmopressin. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554582/
McDowell BD, Wallace RB, Carnahan RM, Chrischilles EA, Lynch CF, Schlechte JA (2011) Demographic differences in incidence for pituitary adenoma. Pituitary 14(1):23–30
Miljic D, Doknic M, Stojanovic M, Nikolic-Djurovic M, Petakov M, Popovic V, Pekic S (2017) Impact of etiology, age and gender on onset and severity of hyponatremia in patients with hypopituitarism: retrospective analysis in a specialised endocrine unit. Endocrine 58(2):312–319
Mindermann T, Wilson CB (1994) Age-related and gender-related occurrence of pituitary adenomas. Clin Endocrinol (Oxf) 41(3):359–364
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535–b2535
Ngo ST, Steyn FJ, McCombe PA (2014) Gender differences in autoimmune disease. Front Neuroendocrinol 35(3):347–369
Nishioka H, Haraoka J, Akada K (2003) Growth potential of prolactinomas in men: is it really different from women? Surg Neurol 59(5):386–390
Osorio RC, Pereira MP, Oh T, Joshi RS, Haddad AF, Pereira KM, Donohue KC, Peeran Z, Carson W, Badani A, Wang EJ, Sudhir S, Chandra A, Jain S, Beniwal A, Gurrola J, El-Sayed IH, Blevins LS, Theodosopoulos PV, Kunwar S, Aghi MK (2023) Correlation between tumor volume and serum prolactin and its effect on surgical outcomes in a cohort of 219 prolactinoma patients. J Neurosurg 138(6):1669–1679
Park SH, Ku CR, Moon JH, Kim EH, Kim SH, Lee EJ (2017) Age- and sex-specific differences as predictors of surgical remission among patients with acromegaly. J Clin Endocrinol Metab 103(3):909–916
Pinzone JJ, Katznelson L, Danila DC, Pauler DK, Miller CS, Klibanski A (2000) Primary medical therapy of micro- and macroprolactinomas in men. J Clin Endocr Metab 85(9):5
Pochon L, Kleinstück FS, Porchet F, Mannion AF (2016) Influence of gender on patient-oriented outcomes in spine surgery. Eur Spine J 25(1):235–246
Richardson DW (1985) Drugs five years later: desmopressin. Ann Intern Med 103(2):228
Rodeghiero F, Castaman G, Mannuccio Mannucci P (1991) Clinical indications for desmopressin (DDAVP) in congenital and acquired von Willebrand disease. Blood Rev 5(3):155–161
Rutkowski MJ, Alward RM, Chen R, Wagner J, Jahangiri A, Southwell DG, Kunwar S, Blevins L, Lee H, Aghi MK (2018) Atypical pituitary adenoma: a clinicopathologic case series. J Neurosurg 128(4):1058–1065
Sanchez-Cardenas C, Fontanaud P, He Z et al (2010) Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci 107(50):21878–21883
Sanno N, Teramoto A, Osamura RY (2000) Long-term surgical outcome in 16 patients with thyrotropin pituitary adenoma. J Neurosurg 93(2):194–200
Schaller B (2003) Gender-related differences in non-functioning pituitary adenomas. Neuro Endocrinol Lett 24(6):425–430
Schaller B (2002) Gender-related differences in growth hormone-releasing pituitary adenomas. A clinicopathological study. Pituitary 5(4):247–53
Serban AL, Sala E, Carosi G, Del Sindaco G, Giavoli C, Locatelli M, Arosio M, Mantovani G, Ferrante E (2019) Recovery of adrenal function after pituitary surgery in patients with Cushing disease: persistent remission or recurrence? Neuroendocrinology 108(3):211–218
Shin SS, Gardner PA, Ng J, Faraji AH, Agarwal N, Chivukula S, Fernandez-Miranda JC, Snyderman CH, Challinor SM (2017) Endoscopic endonasal approach for adrenocorticotropic hormone-secreting pituitary adenomas: outcomes and analysis of remission rates and tumor biochemical activity with respect to tumor invasiveness. World Neurosurg 102:651-658.e1
Siccoli A, Staartjes VE, de Wispelaere MP, Schröder ML (2018) Gender differences in degenerative spine surgery: do female patients really fare worse? Eur Spine J 27(10):2427–2435
Taghvaei M, Sadrehosseini SM, Ardakani JB, Nakhjavani M, Zeinalizadeh M (2018) Endoscopic endonasal approach to the growth hormone–secreting pituitary adenomas: endocrinologic outcome in 68 patients. World Neurosurg 117:e259–e268
Thapar K, Laws ER (2001) Pituitary Surgery. In: Thapar K, Kovacs K, Scheithauer BW, Lloyd RV (eds) Diagnosis and Management of Pituitary Tumors. Humana Press, Totowa, NJ
Tiwari C, Maung E, Gelinne A, Quig N, Thorp B, Zanation A, Ewend M, Sasaki-Adams D, Quinsey C (2022) Disparities in postoperative endocrine outcomes after endoscopic-assisted transsphenoidal pituitary adenoma resection. Cureus. https://doi.org/10.7759/cureus.31934
Trouillas J, Roy P, Sturm N et al (2013) A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol (Berl) 126(1):123–135
Wang SS-Y, Bögli SY, Nierobisch N, Wildbolz S, Keller E, Brandi G (2022) Sex-related differences in patients’ characteristics, provided care, and outcomes following spontaneous intracerebral hemorrhage. Neurocrit Care 37(1):111–120
Wang Z, Guo X, Gao L, Feng C, Lian W, Deng K, Bao X, Feng M, Wang R, Xing B (2019) Delayed remission of growth hormone-secreting pituitary adenoma after transsphenoidal adenectomy. World Neurosurg 122:e1137–e1145
Whitacre CC (2001) Sex differences in autoimmune disease. Nat Immunol 2(9):777–780
Wide L, Hobson BM (1983) Qualitative difference in follicle-stimulating hormone activity in the pituitaries of young women compared to that of men and elderly women*. J Clin Endocrinol Metab 56(2):371–375
Wilson TJ, McKean EL, Barkan AL, Chandler WF, Sullivan SE (2013) Repeat endoscopic transsphenoidal surgery for acromegaly: remission and complications. Pituitary 16(4):459–464
Wolf JM, Cannada L, Van Heest AE, O’Connor MI, Ladd AL (2015) Male and female differences in musculoskeletal disease. J Am Acad Orthop Surg 23(6):339–347
Xu Y, Wan X, Li L, Chen J, Wang J, Shu K, Buchfelder M, Fahlbusch R, Lei T (2022) Extra-pseudocapsular transnasal transsphenoidal resection of pituitary macroadenoma: technique note and evaluation of endocrine function. Curr Med Sci 42(6):1148–1156
Yamada S, Inoshita N, Fukuhara N, Yamaguchi-Okada M, Nishioka H, Takeshita A, Suzuki H, Ito J, Takeuchi Y (2015) Therapeutic outcomes in patients undergoing surgery after diagnosis of Cushing’s disease: a single-center study. Endocr J 62(12):1115–1125
Yasuda ME, Renedo D, Sosa S, Danilowicz K, Recalde R, Zaninovich R, Abbati SG, Cervio A, Giovannini S, Villalonga J, Ulloque-Caamaño L, Reddy K, Socolovsky M, Campero A (2023) Risk factors related to transient diabetes insipidus development following transsphenoidal pituitary adenoma resection: a multicentric study. World Neurosurg 175:e636–e643. https://doi.org/10.1016/j.wneu.2023.03.150. Epub 2023 Apr 7
Yoo F, Chan C, Kuan E, Bergsneider M, Wang M (2018) Comparison of male and female prolactinoma patients requiring surgical intervention. J Neurol Surg Part B Skull Base 79(04):394–400
Zhu H, Li B, Li C, Liu C, Wang X, Gui S, Zhao P, Bai J, Cao L, Zhang Y (2021) The clinical features, recurrence risks and surgical strategies of bone invasive pituitary adenomas. Clin Neurol Neurosurg 201:106455
Zieliński G, Witek P, Maksymowicz M (2015) Outcomes in pituitary surgery in Nelson’s syndrome--therapeutic pitfalls. Endokrynol Pol 66(6):504–13. https://doi.org/10.5603/EP.2015.0062
Funding
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Theiler, S., Hegetschweiler, S., Staartjes, V.E. et al. Influence of gender and sexual hormones on outcomes after pituitary surgery: a systematic review and meta-analysis. Acta Neurochir 165, 2445–2460 (2023). https://doi.org/10.1007/s00701-023-05726-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05726-z