Abstract
This case report concerns a patient suffering from traumatic spinal cord injury with severe spasticity treated with intrathecal baclofen therapy. After revision surgery for a confirmed catheter obstruction, progressive spasticity reappeared. Diagnostics demonstrated signs of catheter fracture or disconnection adjacent to the pump. During revision surgery, the silicone layer surrounding the sutureless pump connector was shown to be curled up, revealing the cause of dysfunction. As far as we know, this form of malconnection has not been reported before. Therefore, surgeons must be aware of this complication and additional inspection of the silicone connector prior to definite connection is advised.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) can lead to extensive limitations in function and quality of life. Spasticity is highly prevalent in SCI and can further increase these limitations. In addition, it can result in pain, contractures, pressure ulcers, and infections which further impairs functioning [7, 9]. Treatment of spasticity is usually first attempted through nonpharmacological treatment such as physiotherapy, after which oral antispasticity drugs such as baclofen are administered if spasticity is widespread [1, 9].
Oral baclofen crosses the blood–brain barrier ineffectively and subsequently has a low intrathecal bioavailability. As a result, oral dosages may need to be high and systemic side effects are common and dose limiting [14]. When oral therapy remains insufficient, intrathecal baclofen (ITB) should be considered next [8, 13]. ITB is administered by a surgically implanted subcutaneous pump with a catheter extending from the pump into the intrathecal space, hereby bypassing the blood–brain barrier resulting in a higher intrathecal bioavailability with a relatively small dosage, ensuing reduced spasticity without the troublesome systemic side effects [8, 9, 13].
Unfortunately, complications after ITB surgery (e.g., drug withdraw, drug overdose, hemorrhage, infection, cerebrospinal fluid (CSF) leakage, pump malfunction, and catheter-related complications) occur frequently and often require re-operation [5, 10]. The most common ITB system-related complications are catheter-related complications (CRC) which comprise kinking, occlusion, migration, fracture, and disconnection and occur in 4–20% of patients within one year after surgery [2, 5].
This paper presents a unique, subtle form of catheter malconnection resulting in the ineffectiveness of a SynchroMed II pump from Medtronic with corresponding Ascenda catheter, in a patient suffering from traumatic SCI-related spasticity. To the best of our knowledge, this complication has not been reported before.
Case report
A 40-year-old male suffered from an incomplete traumatic SCI after a car accident in 2006 resulting in tetraplegia without motor function, but partial intact sensibility below the level of C6. Because of progressive spasticity and contractures resulting in pain and limitations in functioning, an ITB pump was surgically implanted in 2009 and electively revised in 2015 because of end of battery life.
In June 2021, the patient slowly developed intermittent, progressive painful spasticity with frequent spontaneous clonic reflexes in the lower extremities. After excluding other potential causes of progressive spasticity such as constipation or urinary tract infection, an intermittent malfunction of the ITB system was suspected, and an obstruction of the intrathecal part of the catheter was diagnosed through an Indium (In111 DTPA) Single-Photon Emission Computed Tomography (SPECT) study [11]. After diagnosis, oral antispasticity drugs were administered for a short period of time after which spasticity-related symptoms improved. In February 2022, spasticity-related symptoms returned, and the ITB pump including spinal catheter was electively revised in March 2022. Before closure of the wounds, the integrity of the newly implanted system was verified via side port procedure, whereby saline was flushed through the side port into the catheter without any signs of leakage or obstruction.
Postoperative, there were no signs of complications within the first week. However, intermittent painful spasticity accompanied by headaches while seated appeared a week after surgery, highly suspected for recurrent dysfunction. Conventional radiography demonstrated no obvious disconnections, and CSF aspiration was feasible via side port procedure. Subsequently, CT was carried out, whereby contrast was injected into the ITB pump catheter via the catheter access port. This demonstrated catheter patency with the appearance of intrathecal contrast. No extravasation of contrast or visible fluid collections surrounding the catheter or pump that could indicate dysfunction or CSF leakage were observed (Fig. 1). Oral baclofen was given to reduce symptoms, but showed insufficient clinical improvement on spasticity after which a diagnostic intrathecal bolus of 50 mcg baclofen was administered through the pump, without any clinical improvement on spasticity. The next step was an Indium (In111 DTPA) SPECT study. This study demonstrated no intrathecal or intracatheter indium, accompanied by a suspected fluid collection around the pump. After aspiration of the fluid, laboratory tests confirmed the fluid to be CSF and baclofen (Fig. 2). Consequently, elective revision was planned.
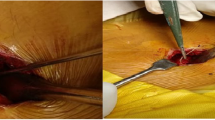
On opening of the abdominal subcutaneous pocket, CSF emerged. After mobilizing and removing the pump for inspection, no disconnection, tear, or active leakage was observed. However, after disconnection of the well-connected and aligned sutureless pump connector (Fig. 3 and 4), the silicone layer surrounding the sutureless pump connector, ensuring watertight connection between the pump and catheter, was shown to be curled up, illuminating the probable cause of dysfunction (Fig. 5). After verifying functionality of the spinal catheter, without signs of dysfunction, the part of the catheter connected to the pump was replaced; after which patency was confirmed through side port procedure and the pump was re-implanted subcutaneously. The postoperative period was uneventful, and the patient was discharged a day after surgery with improvement of spasticity symptoms. Two weeks after surgery, the patient was followed up in the outpatient clinic in accordance with standard postoperative care, without any signs of complications.
Discussion
In this case, ITB system malfunction with CSF leakage was suspected because of recurrent spasticity and headaches after a period of symptom relief directly after elective ITB revision. Following our troubleshooting protocol, dysfunction of the ITB system was confirmed. The cause of ITB dysfunction with CSF leakage around the pump appeared to be a curled up silicone layer surrounding the sutureless pump connector. As far as we know, this complication has not been described before.
As in this case, diagnostics in ITB pump system malfunction can be challenging. The absence of anomalies in conventional radiography, CSF aspiration via the side port, or CT with contrast injection does not preclude ITB system malfunction. If these studies are inconclusive and clinical suspicion of system failure is high, an Indium (In111 DTPA) SPECT-CT study should be obtained [4, 12], whereas an Indium (In111 DTPA) SPECT-CT study slowly infuses the radioisotope and mimics baclofen diffusion, thereby identifying, localizing, and visualizing malfunction with an increased sensitivity and specificity compared to other diagnostics [3, 6].
As described in this case report, the silicone layer surrounding the sutureless pump connector ensuring watertight connection between the pump and catheter was shown to be curled up, demonstrating a unique cause of ITB system dysfunction. Although the cause of malconnection remains unknown, it is conceivable that this occurred before or during connection of the sutureless pump connector to the pump at the time of elective revision in March 2022. Considering this, it is expedient for surgeons to inspect and verify the watertight connection of the sutureless pump connector prior to definite connection.
Conclusion
Complications after ITB surgery are common and multifarious. However, to the best of our knowledge, malconnection as a result of a curled up silicone layer surrounding the sutureless pump connector has not been reported before. Therefore, surgeons must be aware of this potential complication and inspection of the watertight connector prior to definite connection is advised.
References
Adams MM, Hicks AL (2005) Spasticity after spinal cord injury. Spinal Cord 43:577–586. https://doi.org/10.1038/sj.sc.3101757
Bonouvrie LA, Lagendijk KE, Beckerman H, Slot KM, van de Pol LA, Buizer AI (2022) Surgical complications of intrathecal baclofen in children: a single centre, 20-year retrospective cohort study. Eur J Paediatr Neurol 37:94–97. https://doi.org/10.1016/j.ejpn.2022.02.002
Delhaas EM, van Assema DME, Froberg AC, Zwezerijnen B, Harhangi BS, Frankema SPG, Huygen F, van der Lugt A (2021) Isotopic scintigraphy in intrathecal drug delivery failure: a single-institution case series. Neuromodulation 24:1190–1198. https://doi.org/10.1111/ner.13275
Dvorak EM, McGuire JR, Nelson ME (2010) Incidence and identification of intrathecal baclofen catheter malfunction. PM R 2:751–756. https://doi.org/10.1016/j.pmrj.2010.01.016
Feller CN, Awad AJ, Nelson MES, Ketchum N, Pahapill PA (2021) Low rate of intrathecal baclofen pump catheter-related complications: long-term study in over 100 adult patients associated with reinforced catheter. Neuromodulation 24:1176–1180. https://doi.org/10.1111/ner.13412
Fremondiere F, Lacoeuille F, Sher A, Couturier O, Menei P, Richard I, Dinomais M (2016) Isotopic scintigraphy coupled with computed tomography for the investigation of intrathecal baclofen device malfunction. Arch Phys Med Rehabil 97:646–649. https://doi.org/10.1016/j.apmr.2015.10.101
Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB (2017) Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch Phys Med Rehabil 98:1132–1138. https://doi.org/10.1016/j.apmr.2016.09.124
Hugenholtz H, Nelson RF, Dehoux E, Bickerton R (1992) Intrathecal baclofen for intractable spinal spasticity–a double-blind cross-over comparison with placebo in 6 patients. Can J Neurol Sci 19:188–195
Kheder A, Nair KP (2012) Spasticity: pathophysiology, evaluation and management. Pract Neurol 12:289–298. https://doi.org/10.1136/practneurol-2011-000155
Lake W, Shah H (2019) Intrathecal baclofen infusion for the treatment of movement disorders. Neurosurg Clin N Am 30:203–209. https://doi.org/10.1016/j.nec.2018.12.002
Le Breton F, Daviet JC, Monteil J, Vidal J, Munoz M, Dudognon P, Salle JY (2001) Radioisotopic control for baclofen pump catheter failure. Spinal Cord 39:283–285. https://doi.org/10.1038/sj.sc.3101142
Pak S, Jallo GI, Biser A, Ziessman HA (2007) Indium-111 diethylene-triamine-pentaacetic acid scintigraphy in the evaluation of function and patency of baclofen intrathecal infusion systems. Neurosurg Focus 23:E17. https://doi.org/10.3171/foc.2007.23.1.17
Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, Kroin JS (1989) Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320:1517–1521. https://doi.org/10.1056/NEJM198906083202303
Taricco M, Adone R, Pagliacci C, Telaro E (2000) Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev 2000:CD001131. https://doi.org/10.1002/14651858.CD001131
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The manuscript is written in accordance with the 1964 WMA Declaration of Helsinki. All the procedures being performed and treatment received were part of the routine care.
Consent to participate
Informed consent was obtained from the patient for publication of this case report.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koopmans, R.J., Meskers, C.G.M., de Groot, V. et al. Unique form of catheter malconnection following intrathecal baclofen surgery for spinal cord injury: a case report. Acta Neurochir 165, 2707–2710 (2023). https://doi.org/10.1007/s00701-023-05718-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05718-z