Abstract
Purpose
Moyamoya (MM) disease is characterized by progressive intracranial arterial stenosis. Patients commonly need revascularization surgery to optimize cerebral blood flow (CBF). Estimation of CBF and cerebrovascular reserve (CVR) is therefore necessary before and after surgery. However, assessment of CBF before and after indirect revascularization surgery with the multiple burr hole (MBH) technique in MM has not been studied extensively. In this study, we describe our initial experience using arterial spin labeling magnetic resonance perfusion imaging (ASL-MRI) for CBF and CVR assessment before and after indirect MBH revascularization surgery in MM patients.
Methods
Eleven MM patients (initial age 6–50 years, 1 male/10 female) with 19 affected hemispheres were included. A total of 35 ASL-MRI examinations were performed using a 3D-pCASL acquisition before and after i.v. acetazolamide challenge (1000 mg in adults and 10 mg/kg in children). Twelve MBH procedures were performed in seven patients. The first follow-up ASL-MRI was performed 7–21 (mean 12) months after surgery.
Results
Before surgery, CBF was 46 ± 16 (mean ± SD) ml/100 g/min and CVR after acetazolamide challenge was 38.5 ± 9.9 (mean ± SD)% in the most affected territory (middle cerebral artery). In cases in which surgery was not performed, CVR was 56 ± 12 (mean ± SD)% in affected hemispheres. After MBH surgery, there was a relative change in CVR compared to baseline (preop) of + 23.5 ± 23.3% (mean ± SD). There were no new ischemic events.
Conclusion
Using ASL-MRI we followed changes in CBF and CVR in patients with MM. The technique was encouraging for assessments before and after revascularization surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Moyamoya angiopathy (MMA) is a progressive steno-occlusive vascular disease of the terminal internal carotid arteries followed by the development of typical collateral arterial network. Two clinical and pathologic entities are characterized by these morphologic changes: idiopathic moyamoya disease (MMD) and moyamoya “syndrome” (MMS). MMS is associated with a large group of heterogeneous conditions, e.g., certain hematological disorders, congenital anomalies, metabolic disorders, autoimmune diseases, neoplasms, and infectious diseases. MMA leads to disturbance of cerebral blood flow (CBF) and increased risk of ischemic or hemorrhagic stroke [18]. Patients at risk need to undergo revascularization surgery. Several neurosurgical techniques aim to improve CBF either by direct bypass or by various indirect revascularization methods or by combinations thereof [25]. Direct bypass offers immediate improvement of CBF but carries the risk of hyperperfusion syndrome [16] and hemorrhage [14]. Even though severe complications have also been described after indirect revascularization [15], the risks are probably lower with this technique, but the neovascularization takes time. The long-term outcome in both adults and children after indirect revascularization is comparable to or even better than direct methods [26]. Furthermore, indirect revascularization may provide blood flow to more than one vascular territory. The multiple burr hole (MBH) technique of indirect revascularization has been used in both adults and children [2, 8, 22, 23, 27, 35].
A challenge concerns patients with very mild or no symptoms regarding whether they should undergo revascularization surgery or be followed. Several aspects should be considered, such as symptoms, grading of MMA changes (e.g., from digital subtraction angiography (DSA)), presence and size of infarcts/ischemic lesions, assessment of CBF and the cerebrovascular reserve (CVR) after acetazolamide (ACZ) challenge [37]. The gold standard for CBF assessment is 15O-water positron emission tomography (PET), a method with disadvantages such as exposing the patient to radiation, need to use a cyclotron and arterial blood sampling. Arterial spin labeling (ASL) magnetic resonance imaging (MRI) [1, 13, 17, 20, 28, 34] has several advantages for evaluation of CBF in this patient group, especially considering the need for repeated examinations over several years, often in young MM patients. ASL-MRI has a good correlation with [15O]-water PET [12] and single-photon emission computed tomography [28] in MMA. Recently, ASL perfusion was used to study CBF after encephaloduroarteriosynangiosis [19] and bypass procedure [5, 33, 42]. However, evaluation of CVR using ASL-MRI has not been done after indirect revascularization using the MBH technique in MM patients. In this observational study, we assessed longitudinal changes in ASL, both CBF and CVR after acetazolamide challenge, in patients with MMA with and without MBH revascularization surgery.
Patients and methods
Eleven patients (10 female/1 male, age 6–50 years) with MMD admitted to the Department of Neurosurgery at the Uppsala University Hospital, Sweden, 2015–2022 were eligible for this study.
Assessment protocol
The patients had usually been examined with CT angiography or conventional MRI at a local hospital prior to admittance to our department. They were subsequently enrolled in our MMD protocol accordingly. Examinations were performed on a Philips Achieva 3.0 Tesla (Philips Healthcare, Best, the Netherlands) using a 32-channel head coil. A commercially available background suppressed 3D pseudo-continuous ASL (pCASL) gradient spin-echo read-out was acquired with a label duration of 1800 ms and post-label delay of 2500 ms to suppress potential artifacts due to delayed arterial blood flow. Repetition time was 4735 ms, echo time 10.7 ms, spatial resolution 3 × 3 × 6 mm3 and total acquisition time 5 min and 31 s. The labeling plane was placed perpendicular to the brain feeding arteries with the aid of a phase-contrast MR angiography survey, and no flow-crushing gradients were applied. CBF maps were calculated automatically by the scanner according to the model recommended by Alsop et al. [1]. CBF maps were acquired before intravenous injection of ACZ (1 g in adults or 10 mg/kg in children) (baseline) and repeated 5, 15, and 25 min after injection. Dynamic susceptibility contrast (DSC) perfusion using gradient echo-based echo planar imaging was acquired 30 min after ACZ injection. Repetition time was 1392 ms, echo time 29 ms, and spatial resolution 1.72 × 1.72 × 5 mm3. Gadolinium contrast (0.1 mmol/kg) was given at a 5 mL/s injection rate. DSC data were analyzed in NordicICE (NordicNeuroLabs, Bergen, Norway), and included motion correction. Parametric maps of time-to-peak (TTP) were generated. In addition, a structural 3D T2-weighted fluid attenuated inversion recovery (FLAIR) image, 3D contrast-enhanced T1-weighted (CE-T1WI) image and 3D time-of-flight MR angiography were acquired [9, 10].
CBF and TTP maps together with FLAIR images were registered to each patient’s CE-T1WI image. Left and right middle cerebral artery (MCA) territories were delineated by applying the inverse transformation from the vascular MNI template space by spatial normalization of a CE-T1WI image for each patient [9, 10, 30]. Cerebrovascular reserve capacity (CVR) was calculated as CBF after ACZ injection relative to CBF at examination baseline (see below). All the above mentioned processing steps were performed using the SPM12 toolbox (Wellcome Trust Centre for Neuroimaging, London, UK) [9, 10, 30]. In addition to ASL-MRI, most patients were also examined with DSA.
Based on radiological findings and symptoms, patients were either followed with repeated ASL-MRI or planned for revascularization surgery. Criteria for surgery were severe symptoms with repeated ischemic attacks or progressive ischemic symptoms, ischemic lesions on MR, or decreasing and/or low CVR, i.e., either a clear decreasing trend on repeated ASLs and/or CVR around < 30% [10, 40]. Patients with very mild or no symptoms and CVR stable > 30% were monitored (followed). The MMD protocol is outlined in Fig. 1.
Operative procedure
The operative procedure was adapted from the techniques described by Sainte-Rose et al. [35] and later by Lavrysen and Menovsky [24]. All patients were kept on aspirin prophylaxis prior to and during surgery. Patients with bilateral changes were operated on each side on separate occasions; therefore, we did a standard T-shaped incision running in the midline and extending downward to the front of the ear on each side. After subgaleal dissection, the placement of 10–12 burr holes was marked out depending on the most affected region, starting approximately 4 cm from the midline and then in rows downward (Fig. 2). Usually, about three burr holes were placed under the temporal muscle. We carefully planned the locations of the burr holes so that the periosteal flaps would not compromise each other’s blood supply (Fig. 2). After periosteal flap incision, we used a standard 14/11 mm drill bone perforator followed by sharp opening of the dura. The arachnoid and pia mater were incised with a needle (Fig. 2). The periosteal flap was inserted and placed onto the brain surface and fixed with tissue glue (Tisseel®). If possible, we included some muscle with the periosteal flap in the temporal burr holes. A suction drain was used, and the wound was closed. Post-operatively, the patients were well hydrated, and systolic blood pressure was kept at the patients’ normal levels. When patients were fully mobilized in the post-operative ward, they were transferred to a normal ward, usually the following day.
Follow-up
Post-operative clinical follow-up took place 4–6 weeks after surgery, and MR CBF imaging with ACZ challenge was performed at least 7 months postop and then approximately yearly (up to 48 months). Patients deemed not to need surgery were followed (monitored) with clinical examinations and evaluation of hemodynamic reserve every year.
Statistical analysis
CVRC values were calculated based on regional CBF, using the following equation:
for all post-ACZ injection examinations acquired (at 5, 15, and 25 min). The best response was used for further analysis. Values for the three major vascular territories (anterior cerebral artery, MCA, and posterior cerebral artery) were calculated, but only the MCA data are presented. For patients undergoing surgery, the CBF and CVR data obtained most recently prior to surgery were set as “baseline”, and the mean response in change in CBF and CVR was calculated on the basis of all of the patients’ postoperative examinations and related to this baseline as “Relative mean change from baseline in %”. Examinations were grouped into crude time intervals: postop 7–15 months, 22–28 months, and 34–48 months. Continuous data for CBF (ml/100 g/min) and CVR (%) were presented as means ± SD and medians with minimum and maximum ranges. All descriptive statistics were performed using the GraphPad Prism software (Dotmatics, Boston, MA, USA). No further statistical testing was done due to small sample size and heterogeneity of the material.
Results
Patient characteristics are outlined in Table 1. In brief, the median age was 25.5 years, and all cases but one were female. Symptoms ranged from headache to transitory ischemic attacks cerebral infarcts and subarachnoid hemorrhage. Eight cases had classical bilateral MMD and three cases had unilateral findings (in this paper classified as MMS). One of the patients with unilateral changes had neurofibromatosis type 1 (NF1); in the other two patients, the genesis of the unilateral MMA changes was unknown. The median Suzuki stage was 3 (ranging from 2 to 4). In seven patients, surgery was performed using the MBH technique (12 hemispheres), and four patients were monitored without surgery (Table 1).
Overall results
Overall, in operated patients, the mean CBF in the affected MCA territory was calculated to be 46 ± 16 (mean ± SD) ml/100 g/min pre-op and 41.1 ± 12 (mean ± SD) ml/100 g/min at the first post-op ASL examination 7–12 months after surgery (Table 2). In the monitored patients, the mean CBF was 42 ± 12 (mean ± SD) ml/100 g/min. The mean of CVR prior to surgery in all operated cases (i.e., hemispheres) was 38.5 ± 9.9% (mean ± SD) in the affected middle cerebral artery territory (Table 3) vs. 56 ± 12 (mean ± SD)% in the monitored patients (Fig. 3A). The individual preoperative CVR response varied between patients (Table 4); the highest value was observed in patient #1, where the pre-op CVR was 60%, and the lowest pre-op CVR was observed in patient #2 where it was 25%. The actual post-op CVR ranged between 28 and 82% in individual patients (Table 4). In un-operated monitored cases, there were also large variations in CVR, between 39 and 84% (Table 4).
A Graph illustrating the mean pre-op CVR of 38.5 ± 9.9 (mean ± SD) in the middle cerebral artery territory in cases undergoing revascularization surgery with the multiple burr hole technique. Cases we decided to monitor but not operate (followed cases) generally had a higher CVR 56 ± 12%. B and C Comparing the relation between CVR pre- and post-op, the post-op CVR was in general higher compared to baseline. Data from six hemispheres in four patients are included (See Table 4 for details). C Results of relative change of CVR in individual patients for all examinations. These values were then used for mean calculations in each patient presented in Fig. 3B. D Over time, the increase in CVR was maintained after surgery but variability increased. Due to the inhomogeneity of the patients and examinations, no statistical testing between groups was done
In patients with both pre- and post-op examinations (n = 4), we compared the patients’ postoperative examinations (all dates) to patient baseline values (i.e., last CVR prior to surgery), and the mean relative change in CVR after surgery was + 23.5 ± 23.3% (mean ± SD) (Table 3 and Fig. 3B). One patient (#3) showed slightly lower CVR (relative change to baseline of 15 and 21% at 12 and 24 months postoperatively) on the right side (Fig. 3C). The other patients (n = 3) showed a positive relative change in CVR ranging from 4 to 95% postoperatively (Fig. 3C). Studying the CVR over time after MBH surgery, we noticed a sustained positive relative change, but with an increasing variability (Table 5 and Fig. 3D). For details regarding individual patients, see Supplement Fig. 1.
A short description of some illustrative patients included in the study is given below.
Examples of cases revascularized by MBH surgery
Patient #2
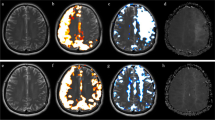
A woman with neurofibromatosis type 1 (NF-1) treated with chemotherapy and radiation at < 6 years of age due to left-sided optic glioma. At age 16, a tumor appeared in the right basal ganglia. A stereotactic biopsy showed pilocytic astrocytoma, and she was treated with gamma knife irradiation at age 18. MRI at this point also showed occlusion of the right ICA and, to lesser degree, of the left M1 segment (Fig. 4). There were no symptoms, and changes were monitored for several years. At age 25, an ASL-MRI with ACZ challenge showed a lower MCA CBF on the occluded right side and a MCA CVR of 25% compared to 43% on the left side. TTP was 8.71 s on the affected right side and 7.59 s on the unaffected side. DSA showed an atypical right-sided MMS with Suzuki grade IV and also a small left-sided ophthalmic aneurysm. A right-sided MBH operation was performed at age 25. A 12-month follow-up, ASL-MRI with ACZ challenge showed that the right-sided CVR had improved to 43% (Fig. 4) and the left-sided CVR to 45%. In addition, contrast-enhanced signs of ingrowth of vessels in burr holes were seen. The post-op TTP was 6.58 s (right) (Fig. 4) and 6.1 s (left). At the latest follow-up at 48 months after surgery, the CVR had decreased somewhat to 28%, and future follow-ups are planned. No focal neurological symptoms have appeared after surgery.
Patient 2 with right-sided MMS changes due to NF1. A Pre-op MRA showing occlusion of the right middle cerebral artery. B Post-op MRI showing contrast enhancement in multiple burr holes indicating ingrowth of blood vessels after MBH surgery. C Pre-op CVR was 25%, and 1 year later, it had increased to 43%. Second follow-up at 27 months showed a CVR decline to 33%, but no new symptoms. D and E Pre-op time-to-peak pre-op was similarly increased to 8.71 s, followed by a decrease to 6.58 s 12 months after surgery
Patient #5
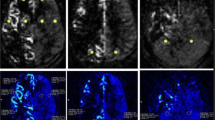
At debut, this 4-year-old girl presented with transient weakness in the right arm. Initial MRI gave suspicion of leukodystrophy (Fig. 5), but a follow-up MR after 12 months was more indicative of chronic ischemia with already some frontal atrophy. DSA showed typical bilateral MMD with a Suzuki grade of II–III (Fig. 5C). She was put on aspirin, and at age 9, she was operated with the MBH-technique bilaterally at 3-month intervals, starting on the left side. Since lengthy MR is not well tolerated in awake young children and we wanted to avoid general anesthesia, CVR was not examined before surgery. However, after surgery, 4 post-op MRI-ASL with ACZ challenge were done with the patient awake. CVR was 4, 10, 14, and 12% on the left side and 19, 17, 25, and 20% on the right side (Fig. 5G). Numerous ingrowths of blood vessels through the burr holes and reduction of chronic ischemia in the white matter were seen on MRI (not shown). Three years after surgery she developed a large intracerebral hemorrhage (Fig. 5D) in the right frontal lobe that needed emergency evacuation through a minimalistic approach. There were no apparent sequelae. DSA was performed to rule out the development of any aneurysm or vascular malformation after revascularization surgery. This could not be seen, but vascular ingrowth through the burr holes and improved capillary perfusion were evident (Fig. 5E).
Patient 5 with bilateral MMD presenting at age 4. A Initial MRI showing chronic ischemia in white matter. B Follow-up MRI with time of flight sequence shows bilateral MMD, confirmed later with C DSA. At age 9, a revascularization procedure with multiple burr holes was performed. Three years after surgery, she developed an intracerebral hemorrhage on the right side D that was evacuated. A DSA was performed showing no aneurysms/AF fistulas. E Ingrowth of numerous blood vessels through the burr holes and improved perfusion were seen on DSA. F The location of the burr holes. G CVR was not established pre-op due to patient age. However, post-op we have been able to follow her CVR, which has generally been low, on the right side about 20% and on the left side between 4–14%. H TTPs has been low but has increased with time post-op
Patient #6
A 26-year-old female presented with headache and transient weakness on her right side. CTA showed bilateral occlusion of the ICA. DSA (Fig. 6) showed bilateral MMD with a Suzuki grade of IV (right) and III (left). ASL-MRI after ACZ challenge showed a CVR of 43% (left) and 42% (right) in the MCA territory bilaterally with no signs of ischemia (Fig. 6C). Due to severe headache and repeated numbness in the right hand and face, a left-sided surgical procedure was carried out 10 months after her initial symptoms. Eight months after the first operation, ASL-MRI with ACZ challenge showed that CVR had increased to 56% on the left side (and to 53% on the right side). A right-sided MBH operation was subsequently performed based upon radiological findings and patient wish. Post-op MRI showed ingrowth of vessels through the burr holes (Fig. 6D).
Patient 6, a 26-year-old female with bilateral MMD. A DSA of right ICA. B TTP map showing delayed TTP bilaterally especially in the MCA territory. C CVR on the left side decreased from 43 to 41% pre-op. We started with a left-sided MBH. Post-op MRI showed improved CVR to 56%, and even on the right side, the CVR improved to 53%. Values are presented in relation to when surgery was performed, on each side separately. However, due to symptoms and a strong patient wish, surgery was also performed on the right side. D Ingrowth of contrast enhancing blood vessels through burr holes after left-sided MBH
Cases managed without surgery
In some cases, surgery was not performed, and patients were monitored based on grading of symptoms, radiological findings and CVR results (Table 4). In general, these cases had better CVRs (ranging from 39 to 84%), no alarming symptoms and no recent ischemic events.
Discussion
In this study, we used ASL-MRI with the ACZ challenge to assess and monitor patients with MMA before and after revascularization surgery with the MBH technique. We report here our experience in 11 patients.
ASL-MRI assessment of MMA patients
MMA patients need to be evaluated regarding symptoms, presence of ischemic lesions as well as their capacity of hemodynamic reserve (CVR). This may require repeated radiological examinations, with considerable drawbacks especially in children, where limitation of exposure to radiation is crucial. Many of these disadvantages are overcome with MRI techniques such as ASL [28, 38] and blood oxygen level-dependent (BOLD) MRI [6]. The ASL technique has recently been used for MMA evaluation in several studies in adults [12, 13] and in children [19, 32]. When ASL-MRI was used to study changes in CBF after revascularization surgery in children with MMD [19, 32], increases in CBF correlated with the degree of collateral formation after superficial temporal artery encephaloduroarteriosynangiosis [19]. After direct revascularization, ASL-MRI has also been used to capture early and late changes in CBF [32-34, 42]. Quon et al. observed a general increase in CBF in the MCA territory, but not in all patients [32]. However, there is a lack of studies using ASL-MRI after MBH revascularization to quantify improvement in CVR. There is one study reporting decreased frontal white-matter MRI-ADC diffusion and improved cognitive flexibility after burr hole surgery [4]. In our assessment of MMA patients, the evaluation included measurements of hemodynamic reserve with ASL-MRI and the ACZ challenge in combination with clinical evaluation of symptoms and findings on conventional diagnostic imaging. The outline of our MMA assessment protocol is summarized in Fig. 1. In principle, if symptoms were mild and CVR was stable and relatively high, patients were monitored, and new ASL-MRI examinations with ACZ challenge were performed at approximately 12- to 18-month intervals. If symptoms were severe or CVR was low, or if there were mild symptoms but a steadily decreasing CVR trend on repeated examinations, patients were selected for revascularization surgery with MBH. The CVR was lower in patients selected for surgery (38.5%) compared to the monitored patients (56%). However, the individual response varied; one surgical patient had a pre-op CVR of 60% (Table 4). This patient was the first in our ASL-MR series, and there were some technical issues related to this first examination, and for this reason, he was not included in pre- vs. post-op comparisons. We therefore believe that regarding patient selection for revascularization surgery, we cannot solely rely on one parameter, instead there must be a combined judgment based on symptoms, presence of ischemic lesions, grading of vasculopathy, CVR and patient wish. Regarding cerebral perfusion after MBH surgery, in this small series, we did not perform any statistical comparing between groups due to the low number of patients and heterogeneous nature of the patient population, but in our descriptive statistics, we noticed a relative improvement in CVR after surgery and the ingrowth of vessels through burr holes indicating an improved cerebrovascular status after MBH surgery. Similar neovascular effects were reported by Sainte-Rose et al. 3 months after MBH [35] and also by Oliviera et al. [29]. Post-op CVR varied considerably, e.g., in one patient the CVR decreased somewhat after surgery. Rao et al. also recently reported a negative effect on CVR in 27% of operated cases [33]. Patients with low CVR improvement after surgery could have a greater risk of new ischemic events [33, 36], and we therefore believe these patients should be followed up for a longer time period.
We did not specifically investigate the degree of vessel ingrowth because the ASL-MRI technique was not sensitive enough. However, based on follow-up MRI-TOF (time of flight) sequences, we saw clear signs of vessel ingrowth in all patients. In a previous MBH study, there was ingrowth of vessels in 41/43 burr holes [23] and in 151 of 160 burr holes using a combined approach of dural inversion and periosteal synangiosis [43]. De Oliveira et al. also noticed significant neoangiogenesis through the burr holes [29]. Thus, this fairly simple and safe surgical technique leads to ingrowth of blood vessels from the external circulation to the brain and improved hemodynamic status in MMA. In the future, we need to go more into detail regarding differences between children and adults in the extent of neovascularization after MBH, but this was not within the scope of the present study, instead we wanted to describe our initial experiences using ASL-MRI as an evaluation tool in MMD surgery.
Overall clinical outcome
After surgery, there was an overall improvement in symptoms, and no new ischemic events were seen in any patient. Also, no other serious events such as CSF leakage or subdural hematoma were seen, despite the usage of perioperative aspirin. We had one serious intracerebral hemorrhagic event in a young girl 3 years after surgery, but she recovered fully after surgical evacuation of the hematoma. This patient was subsequently examined with a post-op DSA that showed no vessel malformation/AV shunting after surgery. Instead, numerous new vessels had been formed through burr holes, and there was improved cerebral capillary perfusion bilaterally. We did not routinely examine all patients with post-op DSA since we did not think this was indicated because the patients had no ischemic symptoms, instead they were monitored with ASL-MRI including the ACZ challenge.
Methodological considerations
Some methodological considerations regarding the MRI technique need to be discussed. Thus, it is obvious that all the present techniques for quantification of the hemodynamic status of CBF and CVR have their strengths and limitations. In this diverse group of moyamoya patients, the research field is striving to identify the most effective MRI techniques for characterizing cerebral perfusion; however, the most appropriate method has not finally been established. Ultimately, the aim is to adopt a safe and reliable quantitative approach to measure cerebral vascular reserve and cerebral perfusion. An issue regarding the current recommended implementation of ASL, with single delay-based acquisitions, is the inherent dependency on arterial transit time. Arterial transit time artifacts affecting CBF quantification may appear in patients with slow blood flow [39, 41] and are commonly seen in patients with cerebrovascular diseases such as MMS. We have previously demonstrated that overestimation of the effects of arterial transit time artifacts does not significantly affect CBF measurements [9]. However, in the presence of severely delayed arterial blood flow, underestimation of CBF may occur when no or little magnetically labeled blood has reached the imaging volume in time for the readout. The extent of these effects is dependent on the severity of the delay flow and the post-label delay used during the ASL acquisition [11]. To mitigate the underestimation of the CBF, we used a post-label delay of 2500 ms in the present study. However, the drawback is a decreased signal-to-noise ratio due to the T1 relaxation of arterial blood. All ASL datasets included in this study were reviewed visually to exclude vascular territories with a severe delay-induced underestimation of CBF, and no exclusions were made. Still, there may be vascular territories that can be moderately affected.
Recently, multi-delay-based ASL approaches have gained a more widespread application and are recommended in several reports as the first choice over single delay-based ASL in patients with cerebrovascular diseases, if available [20]. Recent studies have shown the potential of CBF and CVR measurements using both single delay- and multi-delay-based ASL for follow-up in MM [11, 12, 19, 32, 33, 42, 44].
Velocity-selective ASL (VSASL) is a novel ASL-based method that is insensitive to delayed arterial blood flow and reduced ASL signal decay due to T1 relaxation [31]. In a recent study, Bolar et al. demonstrated the promising potential of VS-ASL in comparison to pulsed ASL in pediatric patients with MM [3]. However, pulsed ASL is inferior in comparison to pCASL in terms of the signal-to-noise ratio. Moreover, implementation of VSASL has challenges such as sensitivity to eddy currents, electromagnetic field in-homogeneities, diffusion attenuation, patient movement and CSF contamination, the last an issue which becomes more problematic in the aging brain [31]. However, both multi-delay ASL and VSASL have very limited commercial availability, which affects their accessibility.
Another method to estimate CVR is the BOLD MRI technique, exploiting the difference in MRI signal between oxyhemoglobin and deoxyhemoglobin, oxyhemoglobin having higher signal. An increase in blood flow will thus cause increased BOLD signal. Using this technique, the CVR has been assessed with breath-holding or inhaling of carbon dioxide as a vasoactive stimulus [6, 7]. A major drawback with this technique, however, is its qualitative nature that does not permit quantitative measures of blood flow. Another limitation is that the BOLD signal has a multifactorial origin that depends on blood flow as well as other factors, e.g., CMRO2 [21].
Limitations of the study
This study has several limitations related to the low number of included patients and their heterogeneity with regard to age and pathology; therefore, the results have to be interpreted with caution. Further studies including more patients will be needed to corroborate our findings.
Conclusions
We believe that a combined judgment based on different radiological modalities, such as ASL-MRI with ACZ challenge, together with clinical examinations offers good possibilities to decide the timing of indirect revascularization of MMA patients, thereby limiting revascularization surgery to patients that have a clear need for it.
Data Availability
The datasets generated during and/or analyzed dur ing the current study are available from the corresponding author on reasonable request.
References
Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116. https://doi.org/10.1002/mrm.25197
Blauwblomme T, Mathon B, Naggara O, Kossorotoff M, Bourgeois M, Puget S, Meyer P, Brousse V, de Montalembert M, Brunelle F, Zerah M, Sainte-Rose C (2017) Long-term outcome after multiple burr hole surgery in children with moyamoya angiopathy: a single-center experience in 108 hemispheres. Neurosurgery 80:950–956. https://doi.org/10.1093/neuros/nyw161
Bolar DS, Gagoski B, Orbach DB, Smith E, Adalsteinsson E, Rosen BR, Grant PE, Robertson RL (2019) Comparison of CBF measured with combined velocity-selective arterial spin-labeling and pulsed arterial spin-labeling to blood flow patterns assessed by conventional angiography in pediatric moyamoya. AJNR Am J Neuroradiol 40:1842–1849. https://doi.org/10.3174/ajnr.A6262
Calviere L, Loubiere P, Planton M, Cazzola V, Catalaa I, Mirabel H, Sol JC, Bonneville F (2020) Decreased frontal white-matter diffusion and improved cognitive flexibility after burr-hole surgery in moyamoya angiopathy. BMC Neurol 20:30. https://doi.org/10.1186/s12883-020-1614-x
Cui B, Zhang T, Ma Y, Chen Z, Ma J, Ma L, Jiao L, Zhou Y, Shan B, Lu J (2020) Simultaneous PET-MRI imaging of cerebral blood flow and glucose metabolism in the symptomatic unilateral internal carotid artery/middle cerebral artery steno-occlusive disease. Eur J Nucl Med Mol Imaging 47:1668–1677. https://doi.org/10.1007/s00259-019-04551-w
Dlamini N, Shah-Basak P, Leung J, Kirkham F, Shroff M, Kassner A, Robertson A, Dirks P, Westmacott R, deVeber G, Logan W (2018) Breath-hold blood oxygen level-dependent MRI: a tool for the assessment of cerebrovascular reserve in children with moyamoya disease. AJNR Am J Neuroradiol 39:1717–1723. https://doi.org/10.3174/ajnr.A5739
Dlamini N, Slim M, Kirkham F, Shroff M, Dirks P, Moharir M, MacGregor D, Robertson A, deVeber G, Logan W (2020) Predicting ischemic risk using blood oxygen level-dependent MRI in children with moyamoya. AJNR Am J Neuroradiol 41:160–166. https://doi.org/10.3174/ajnr.A6324
Endo M, Kawano N, Miyaska Y, Yada K (1989) Cranial burr hole for revascularization in moyamoya disease. J Neurosurg 71:180–185. https://doi.org/10.3171/jns.1989.71.2.0180
Fahlstrom M, Lewen A, Enblad P, Larsson EM, Wikstrom J (2020) High intravascular signal arterial transit time artifacts have negligible effects on cerebral blood flow and cerebrovascular reserve capacity measurement using single postlabel delay arterial spin-labeling in patients with moyamoya disease. AJNR Am J Neuroradiol 41:430–436. https://doi.org/10.3174/ajnr.A6411
Fahlstrom M, Wikstrom J, Borota L, Enblad P, Lewen A (2021) Variable temporal cerebral blood flow response to acetazolamide in moyamoya patients measured using arterial spin labeling. Front Neurol 12:615017. https://doi.org/10.3389/fneur.2021.615017
Fan AP, Guo J, Khalighi MM, Gulaka PK, Shen B, Park JH, Gandhi H, Holley D, Rutledge O, Singh P, Haywood T, Steinberg GK, Chin FT, Zaharchuk G (2017) Long-delay arterial spin labeling provides more accurate cerebral blood flow measurements in moyamoya patients: a simultaneous positron emission tomography/MRI study. Stroke 48:2441–2449. https://doi.org/10.1161/STROKEAHA.117.017773
Fan AP, Khalighi MM, Guo J, Ishii Y, Rosenberg J, Wardak M, Park JH, Shen B, Holley D, Gandhi H, Haywood T, Singh P, Steinberg GK, Chin FT, Zaharchuk G (2019) Identifying hypoperfusion in moyamoya disease with arterial spin labeling and an [(15)O]-water positron emission tomography/magnetic resonance imaging normative database. Stroke 50:373–380. https://doi.org/10.1161/STROKEAHA.118.023426
Federau C, Christensen S, Zun Z, Park SW, Ni W, Moseley M, Zaharchuk G (2017) Cerebral blood flow, transit time, and apparent diffusion coefficient in moyamoya disease before and after acetazolamide. Neuroradiology 59:5–12. https://doi.org/10.1007/s00234-016-1766-y
Fujimura M, Shimizu H, Mugikura S, Tominaga T (2009) Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 71:223–227; discussion 227. https://doi.org/10.1016/j.surneu.2007.07.077
Fujimura M, Kaneta T, Shimizu H, Tominaga T (2009) Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg Rev 32:245–249; discussion 249. https://doi.org/10.1007/s10143-009-0184-6
Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T (2007) Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol 67:273–282. https://doi.org/10.1016/j.surneu.2006.07.017
Goetti R, O’Gorman R, Khan N, Kellenberger CJ, Scheer I (2013) Arterial spin labelling MRI for assessment of cerebral perfusion in children with moyamoya disease: comparison with dynamic susceptibility contrast MRI. Neuroradiology 55:639–647. https://doi.org/10.1007/s00234-013-1155-8
Guey S, Tournier-Lasserve E, Herve D, Kossorotoff M (2015) Moyamoya disease and syndromes: from genetics to clinical management. Appl Clin Genet 8:49–68. https://doi.org/10.2147/TACG.S42772
Ha JY, Choi YH, Lee S, Cho YJ, Cheon JE, Kim IO, Kim WS (2019) Arterial spin labeling MRI for quantitative assessment of cerebral perfusion before and after cerebral revascularization in children with moyamoya disease. Korean J Radiol 20:985–996. https://doi.org/10.3348/kjr.2018.0651
Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X (2016) Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 281:337–356. https://doi.org/10.1148/radiol.2016150789
Juttukonda MR, Donahue MJ (2019) Neuroimaging of vascular reserve in patients with cerebrovascular diseases. Neuroimage 187:192–208. https://doi.org/10.1016/j.neuroimage.2017.10.015
Kapu R, Symss NP, Cugati G, Pande A, Vasudevan CM, Ramamurthi R (2010) Multiple burr hole surgery as a treatment modality for pediatric moyamoya disease. J Pediatr Neurosci 5:115–120. https://doi.org/10.4103/1817-1745.76102
Kawaguchi T, Fujita S, Hosoda K, Shose Y, Hamano S, Iwakura M, Tamaki N (1996) Multiple burr-hole operation for adult moyamoya disease. J Neurosurg 84:468–476. https://doi.org/10.3171/jns.1996.84.3.0468
Lavrysen E, Menovsky T (2019) How I do it: operative nuances of multiple burr hole surgery for moyamoya disease and syndrome. Acta Neurochir (Wien) 161:171–175. https://doi.org/10.1007/s00701-018-3743-1
Liu JJ, Steinberg GK (2017) Direct versus indirect bypass for moyamoya disease. Neurosurg Clin N Am 28:361–374. https://doi.org/10.1016/j.nec.2017.02.004
Macyszyn L, Attiah M, Ma TS, Ali Z, Faught R, Hossain A, Man K, Patel H, Sobota R, Zager EL, Stein SC (2017) Direct versus indirect revascularization procedures for moyamoya disease: a comparative effectiveness study. J Neurosurg 126:1523–1529. https://doi.org/10.3171/2015.8.JNS15504
Mirone G, Cicala D, Meucci C, d’Amico A, Santoro C, Muto M, Cinalli G (2019) Multiple burr-hole surgery for the treatment of moyamoya disease and quasi-moyamoya disease in children: preliminary surgical and imaging results. World Neurosurg 127:e843–e855. https://doi.org/10.1016/j.wneu.2019.03.282
Noguchi T, Kawashima M, Irie H, Ootsuka T, Nishihara M, Matsushima T, Kudo S (2011) Arterial spin-labeling MR imaging in moyamoya disease compared with SPECT imaging. Eur J Radiol 80:e557-562. https://doi.org/10.1016/j.ejrad.2011.01.016
Oliveira RS, Amato MC, Simao GN, Abud DG, Avidago EB, Specian CM, Machado HR (2009) Effect of multiple cranial burr hole surgery on prevention of recurrent ischemic attacks in children with moyamoya disease. Neuropediatrics 40:260–264. https://doi.org/10.1055/s-0030-1249069
Petr J, Schramm G, Hofheinz F, Langner J, van den Hoff J (2013) Partial volume correction in arterial spin labeling using a Look-Locker sequence. Magn Reson Med 70:1535–1543. https://doi.org/10.1002/mrm.24601
Qin Q, Alsop DC, Bolar DS, Hernandez-Garcia L, Meakin J, Liu D, Nayak KS, Schmid S, van Osch MJP, Wong EC, Woods JG, Zaharchuk G, Zhao MY, Zun Z, Guo J, Group ISS (2022) Velocity-selective arterial spin labeling perfusion MRI: a review of the state of the art and recommendations for clinical implementation. Magn Reson Med 88:1528–1547. https://doi.org/10.1002/mrm.29371
Quon JL, Kim LH, Lober RM, Maleki M, Steinberg GK, Yeom KW (2019) Arterial spin-labeling cerebral perfusion changes after revascularization surgery in pediatric moyamoya disease and syndrome. J Neurosurg Pediatr 23:486–492. https://doi.org/10.3171/2018.11.PEDS18498
Rao VL, Prolo LM, Santoro JD, Zhang M, Quon JL, Jin M, Iyer A, Yedavalli V, Lober RM, Steinberg GK, Yeom KW, Grant GA (2022) Acetazolamide-challenged arterial spin labeling detects augmented cerebrovascular reserve after surgery for moyamoya. Stroke 53:1354–1362. https://doi.org/10.1161/STROKEAHA.121.036616
Saida T, Masumoto T, Nakai Y, Shiigai M, Matsumura A, Minami M (2012) Moyamoya disease: evaluation of postoperative revascularization using multiphase selective arterial spin labeling MRI. J Comput Assist Tomogr 36:143–149. https://doi.org/10.1097/RCT.0b013e31824150dd
Sainte-Rose C, Oliveira R, Puget S, Beni-Adani L, Boddaert N, Thorne J, Wray A, Zerah M, Bourgeois M (2006) Multiple bur hole surgery for the treatment of moyamoya disease in children. J Neurosurg 105:437–443. https://doi.org/10.3171/ped.2006.105.6.437
So Y, Lee HY, Kim SK, Lee JS, Wang KC, Cho BK, Kang E, Lee DS (2005) Prediction of the clinical outcome of pediatric moyamoya disease with postoperative basal/acetazolamide stress brain perfusion SPECT after revascularization surgery. Stroke 36:1485–1489. https://doi.org/10.1161/01.STR.0000170709.95185.b1
Thines L, Petyt G, Aguettaz P, Bodenant M, Himpens FX, Lenci H, Henon H, Gauthier C, Hossein-Foucher C, Cordonnier C, Lejeune JP (2015) Surgical management of Moyamoya disease and syndrome: current concepts and personal experience. Rev Neurol (Paris) 171:31–44. https://doi.org/10.1016/j.neurol.2014.08.007
Uchihashi Y, Hosoda K, Zimine I, Fujita A, Fujii M, Sugimura K, Kohmura E (2011) Clinical application of arterial spin-labeling MR imaging in patients with carotid stenosis: quantitative comparative study with single-photon emission CT. AJNR Am J Neuroradiol 32:1545–1551. https://doi.org/10.3174/ajnr.A2525
Ukai R, Mikami T, Nagahama H, Wanibuchi M, Akiyama Y, Miyata K, Mikuni N (2020) Arterial transit artifacts observed by arterial spin labeling in Moyamoya disease. J Stroke Cerebrovasc Dis 29:105058. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105058
Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M (2009) The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 30:876–884. https://doi.org/10.3174/ajnr.A1538
Zaharchuk G (2012) Arterial spin labeling for acute stroke: practical considerations. Transl Stroke Res 3:228–235. https://doi.org/10.1007/s12975-012-0159-8
Zhao MY, Armindo RD, Gauden AJ, Yim B, Tong E, Moseley M, Steinberg GK, Zaharchuk G (2022) Revascularization improves vascular hemodynamics - a study assessing cerebrovascular reserve and transit time in Moyamoya patients using MRI. J Cereb Blood Flow Metab 271678X221140343. https://doi.org/10.1177/0271678X221140343
Zhao X, Wang C, Ji Y, Han C, Wang M (2015) Therapeutic effect of multiple burr hole operation combined with dural inversion and periosteal synangiosis for moyamoya disease. Br J Neurosurg 29:811–817. https://doi.org/10.3109/02688697.2015.1071318
Zhao MY, Fan AP, Chen DY, Ishii Y, Khalighi MM, Moseley M, Steinberg GK, Zaharchuk G (2022) Using arterial spin labeling to measure cerebrovascular reactivity in Moyamoya disease: insights from simultaneous PET/MRI. J Cereb Blood Flow Metab 42:1493–1506. https://doi.org/10.1177/0271678X221083471
Funding
Open access funding provided by Uppsala University. The Swedish Stroke Association and the Erik, Karin and Gösta Selander’s foundation, Uppsala, Sweden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the regional Ethical Review Board. All procedures performed involving humans were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the patients or children’s parents.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lewén, A., Fahlström, M., Borota, L. et al. ASL-MRI-guided evaluation of multiple burr hole revascularization surgery in Moyamoya disease. Acta Neurochir 165, 2057–2069 (2023). https://doi.org/10.1007/s00701-023-05641-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05641-3