Abstract
Epileptic seizure is the common symptom associated with lipomas in the Sylvian fissure (Sylvian lipomas). Removal of these lipomas carries risks of hemorrhage and brain damage. We report a surgical strategy of not removing the lipoma in a case of intractable temporal lobe epilepsy associated with Sylvian lipoma. We performed anterior temporal lobectomy with preservation of the pia mater of the Sylvian fissure and achieved seizure freedom. Focal cortical dysplasia type 1 of the epileptic neocortex adjacent to the Sylvian lipoma was pathologically diagnosed. We recommend our surgical procedure in similar cases to avoid complications and achieve adequate seizure control.
Similar content being viewed by others
Introduction
Intracranial lipomas are congenital malformations that occur in 0.46–1% of intracranial tumors [3]. These lipomas are primarily localized to the interhemispheric area, followed by localization to the dorsal brain stem [19], with the majority of them not causing life-threatening symptoms [16]. Removal of the lipomas may result in postoperative hemorrhage and brain parenchymal damage, owing to the strong attachment to the surrounding tissue [5, 16]. Therefore, no consensus exists on surgical indication for the removal of intracranial lipomas in most cases.
Lipomas in the Sylvian fissure (Sylvian lipomas) are extremely rare, with incidences of 3.4% and 5% in case series of intracranial lipomas by Maiuri et al. [11] and Truwit et al. [19], respectively. Sylvian lipomas are clinically associated with epileptic seizures, particularly focal impaired awareness seizures (FIAS) [5]. In some cases, the seizures are refractory to antiepileptic drugs, requiring the consideration of surgical treatment for the epilepsy [1]. The accurate identification of the epileptic focus and prevention of complications are the two most important factors for the safe and effective surgical treatment of patients with temporal lobe epilepsy (TLE) due to Sylvian lipoma. Here, we attempted a strategy of identifying the epileptic focus using intraoperative electrocorticography (ioECoG) and preventing complications by not removing the lipoma during the removal of the epileptic region.
Case presentation
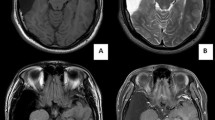
A 19-year-old woman had FIAS presented with an aura of olfactory hallucinations, followed by behavior arrest with prominent oroalimentary automatism. She had no history of having focal to bilateral tonic–clonic seizures. Her FIAS was not controlled for more than 2 years even with 1000 mg of levetiracetam (LEV), with a frequency of one seizure per week. Her language dominancy was left (right-handed) on The Edinburgh Handedness Inventory Test [13]. Wechsler Memory Scale-Revised (WMS-R) demonstrated no memory decline (verbal memory 94, visual memory 103, general memory 96, attention/concentration 105, delayed recall 96) before surgery. Magnetic resonance imaging (MRI) showed a mass lesion in the left Sylvian fissure with homogeneous hyperintensity on both T1-weighted and T2-weighted images but no diffusion restriction on diffusion-weighted images/apparent diffusion coefficient maps (Fig. 1). Fluid-attenuated inversion recovery images demonstrated normal signal in the hippocampus. Fluorodeoxyglucose positron emission tomography revealed severe focal hypometabolism in the left anterior temporal lobe. Preoperative long-term scalp video-EEG monitoring (EEG-1200, Nihon Kohden, Tokyo, Japan) using the 10–20 international system for 3 days did not show ictal discharges but detected interictal epileptiform discharges (IEDs) at T1 and F7 electrodes with reproducibility. We concluded that the patient’s epilepsy was medically intractable and a surgical intervention for the TLE was needed. We applied the surgical strategy of identifying the epileptic focus using ioECoG and resecting it without removal of the Sylvian lipoma.
The Sylvian lipoma shows a markedly high signal intensity on axial T1-weighted imaging (A) and fluid-attenuated inversion recovery imaging (B), along with a slightly high signal intensity on axial T2-weighted imaging (C) on preoperative magnetic resonance imaging. The Sylvian lipoma shows signal supression on axial diffusion-weighted imaging (D) and the corresponding apparent diffusion coefficient map (E). The anterior temporal cortex adjacent to the Sylvian lipoma shows hypometabolism on fluorodeoxyglucose positron emission tomography/computed tomography (F)
After the pterional craniotomy, subpial dissection was initiated along the upper border of the superior temporal gyrus to protect the pia mater of the Sylvian fissure and approached to the inferior horn of the lateral ventricle. After obtaining an adequate space to observe the amygdala and the hippocampus, a 10-min ioECoG was recorded on the surface of the amygdala, hippocampus, and anterior temporal lateral cortex adjacent to the Sylvian lipoma using platinum electrodes (Unique Medical, Tokyo, Japan). The ioECoG showed IEDs at the strip electrodes located on the anterior lateral temporal lobe (Fig. 2). Therefore, we performed the anterior temporal lobectomy (ATL) 3 cm from the temporal tip without removing the Sylvian lipoma (Fig. 3). Postoperative MRI demonstrated the preserved Sylvian lipoma and branches of the middle cerebral artery within the Sylvian fissure (Fig. 4). Histopathological examination of the neocortical specimen of the anterior temporal lobe revealed neuronal cytoarchitectural abnormalities in the cortex. Thus, focal cortical dysplasia (FCD) type 1 of the anterior temporal neocortex was diagnosed.
At 2 years postoperatively, she never had seizures with 1000 mg of LEV without any neurological sequelae. Furthermore, follow-up EEG found no IEDs, while WMS-R 6 months after surgery indicated no memory decline (verbal memory 105, visual memory 114, general memory 99, attention/concentration 103, delayed recall 108).
Discussion
Detection of epileptic focus using ioECoG in TLE due to Sylvian lipoma
Based on the ioECoG findings, we concluded that the neocortex adjacent to the Sylvian lipoma was the epileptic focus. Consequently, ATL with the preservation of the Sylvian lipoma was performed that resulted in seizure freedom.
Temporal lobe tumors, particularly low-grade gliomas and glioneuronal tumors, are common causes of intractable TLE and consequently have been described as long-term epilepsy-associated tumors (LEATs) [20]. One of the surgical strategies for TLE with LEATs includes gross total tumor resection alone for seizure control; however, an adequate surgical strategy for TLE with LEATs has not been fully established [12]. Jooma et al. reported that the resection of the mesial temporal structures is an important factor for surgical success in refractory TLE because both the lesion and hippocampus could have increased epileptogenicity [8]. In another study, Sugano et al. evaluated the efficacy of the additional removal of electrically positive foci using ioECoG in cases of TLE with LEATs, and they concluded that ioECoG monitoring was valuable for detecting epileptic foci of the mesial temporal structures [18]. However, the contribution of the peritumoral tissue to the epileptogenicity also remains unclear [8], although Pelliccia et al. and Giulioni et al. reported a relationship between epilepsy and tumor-associated FCD [6, 14]. Nowak et al. found that extended temporal lobe resection provided for optimal resection of the epileptogenic tissue by removing the invisible foci of highly epileptogenic cortical dysplasia adjacent to the tumors [12].
Sylvian lipomas are extramedullary lesions; thus, an epileptic mechanism of LEATs may not be applicable in TLEs with Sylvian lipoma. Moreover, in this case, the epilepsy may have been caused by invisible foci of cortical dysplasia as MRI-negative TLE with no relation to the lipoma [15]. However, a lipoma is thought to be an aberrant tissue of the meninx primitiva, the mesenchymal precursor of the leptomeninges, formed during the development of the subarachnoid cisterns. Thus, the formation of a cerebral lipoma might be part of a complex malformation that also involves vascular abnormalities and abnormal cortical development within its vicinity [5, 9, 16]. Kakita et al. reported a polymicrogyric appearance in the cerebral cortex underlying the lipoma and demonstrated that focal disturbances in cortical development occur in association with the development of lipomas [9]. The abnormalities of the cortical laminae could be an etiology of epilepsy, which is supported by the histopathological diagnosis in our case. The epileptic focus in patients with Sylvian lipoma could be in the mesial temporal cortex or the variety of associated with neocortical abnormalities [5, 9, 16] In surgical cases of TLE due to Sylvian lipoma, we propose the strategy of detecting the epileptic focus within the mesial temporal structures and surrounding neocortical tissues using ioECoG.
Surgical technique for preventing complication and achieving satisfactory seizure outcome
In cases where the cortex underlying the lipoma is evaluated to be the epileptic focus, the decision to remove the lipoma remains controversial. Surgical excision of intracranial lipomas is associated with serious hemorrhagic complications and the unfavorable seizure outcome [2, 4, 7, 11]. The tight adherence of the lipoma to the adjacent cortical and intervening vascular structures is a crucial factor that makes surgical excision difficult [5].
Dermoid cysts in the temporal lobe are another type of extramedullary benign tumor that can cause intractable TLE [17]. Li et al. reported a case of a dermoid cyst in the Sylvian fissure and discussed that the lipid content in the dermoid cyst could cause chemical irritation or meningitis and consequent epileptic discharge [10]. Hence, cyst rupture may be a risk factor for new-onset seizures in patients with Sylvian fissure dermoid cyst. We considered this possibility in our lipoma case as well. Thus, if we damaged the fibrous capsule surrounding the lipoma, secondary epileptogenic activity due to the lipid content might have developed in our case. In surgical cases of TLE due to Sylvian lipoma, we propose the surgical strategy of not removing the lipoma for avoiding irritation or meningitis caused by its lipid content and hemorrhagic complications associated with its strong adhesion to the surrounding tissue. Subpial dissection could be an appropriate technique to preserve the pia mater of the Sylvian fissure, thereby preventing damage to the Sylvian lipoma.
Conclusion
We reported a surgical strategy using ioECoG to identify the epileptic focus in the TLE due to Sylvian lipoma for achieving favorable seizure outcome. Furthermore, with regard to th resection of the epileptic focus, ATL with subpial dissection to protect the Sylvian fissure and isolate the lipoma might be a reasonable surgical technique considering the lipoma formation and presence of FCD type 1 changes in the adjacent cortex.
Abbreviations
- ATL:
-
Anterior temporal lobectomy
- EEG:
-
Electroencephalography
- FCD:
-
Focal cortical dysplasia
- FIAS:
-
Focal impaired awareness seizures
- IEDs:
-
Interictal epileptiform discharges
- ioECoG:
-
Intraoperative electrocorticography
- LEATs:
-
Long-term epilepsy-associated tumors
- LEV:
-
Levetiracetam
- MRI:
-
Magnetic resonance imaging
- TLE:
-
Temporal lobe epilepsy
- WMS-R:
-
Wechsler Memory Scale-Revised
References
Aguirre MEE, de Lapiscina EHM (2014) Lipoma: an overview. Tumors of the Central Nervous System 13: 223–229.
Cherian A, Baheti NN, Menon R, Iyer RS (2011) Hemispheric intracranial lipoma with seizure: look under the carpet. Neurol India 59:128–130. https://doi.org/10.4103/0028-3886.76865
Donati F, Vassella F, Kaiser G, Blumberg A (1992) Intracranial lipomas. Neuropediatrics 23:32–38. https://doi.org/10.1055/s-2008-1071309
Eghwrudjakpor PO, Kurisaka M, Fukuoka M, Mori K (1991) Intracranial lipomas. Acta Neurochir (Wien) 110:124–128. https://doi.org/10.1007/bf01400679
Feldman RP, Marcovici A, LaSala PA (2001) Intracranial lipoma of the sylvian fissure. Case report and review of the literature. J Neurosurg 94:515–519. https://doi.org/10.3171/jns.2001.94.3.0515
Giulioni M, Rubboli G, Marucci G, Martinoni M, Volpi L, Michelucci R, Marliani AF, Bisulli F, Tinuper P, Castana L, Sartori I, Calbucci F (2009) Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg 111:1275–1282. https://doi.org/10.3171/2009.3.Jns081350
Guye M, Gastaut JL, Bartolomei F (1999) Epilepsy and perisylvian lipoma/cortical dysplasia complex. Epileptic Disord 1:69–73
Jooma R, Yeh HS, Privitera MD, Gartner M (1995) Lesionectomy versus electrophysiologically guided resection for temporal lobe tumors manifesting with complex partial seizures. J Neurosurg 83:231–236. https://doi.org/10.3171/jns.1995.83.2.0231
Kakita A, Inenaga C, Kameyama S, Masuda H, Ueno T, Honma J, Shimohata M, Takahashi H (2005) Cerebral lipoma and the underlying cortex of the temporal lobe: pathological features associated with the malformation. Acta Neuropathol 109:339–345. https://doi.org/10.1007/s00401-004-0955-7
Li Q, You C, Zan X, Chen N, Zhou L, Xu J (2012) Mature cystic teratoma (dermoid cyst) in the Sylvian fissure: a case report and review of the literature. J Child Neurol 27:211–217. https://doi.org/10.1177/0883073811415681
Maiuri F, Cirillo S, Simonetti L, De Simone MR, Gangemi M (1988) Intracranial lipomas. Diagnostic and therapeutic considerations. J Neurosurg Sci 32:161–167
Nowak A, Rysz A, Dziedzic T, Czernicki T, Kunert P, Maj E, Marchel A (2019) Predictors of class I epilepsy surgery outcome in tumour-related chronic temporal lobe epilepsy in adults. Neurol Neurochir Pol 53:466–475. https://doi.org/10.5603/PJNNS.a2019.0061
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. https://doi.org/10.1016/0028-3932(71)90067-4
Pelliccia V, Deleo F, Gozzo F, Sartori I, Mai R, Cossu M, Tassi L (2017) Early and late epilepsy surgery in focal epilepsies associated with long-term epilepsy-associated tumors. J Neurosurg 127:1147–1152. https://doi.org/10.3171/2016.9.Jns161176
Ronan L, Scanlon C, Murphy K, Maguire S, Delanty N, Doherty CP, Fitzsimons M (2011) Cortical curvature analysis in MRI-negative temporal lobe epilepsy: a surrogate marker for malformations of cortical development. Epilepsia 52:28–34. https://doi.org/10.1111/j.1528-1167.2010.02895.x
Saatci I, Aslan C, Renda Y, Besim A (2000) Parietal lipoma associated with cortical dysplasia and abnormal vasculature: case report and review of the literature. AJNR Am J Neuroradiol 21:1718–1721
Sugano H, Shimizu H, Sunaga S, Arai N, Tamagawa K (2006) Temporal lobe epilepsy caused by dermoid cyst. Neurol Med Chir (Tokyo) 46:206–209. https://doi.org/10.2176/nmc.46.206
Sugano H, Shimizu H, Sunaga S (2007) Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure 16:120–127
Truwit CL, Barkovich AJ (1990) Pathogenesis of intracranial lipoma: an MR study in 42 patients. AJR Am J Roentgenol 155:855–864; discussion 865. https://doi.org/10.2214/ajr.155.4.2119122
Vogt VL, Witt JA, Delev D, Grote A, von Lehe M, Becker AJ, Schramm J, Elger CE, Helmstaedter C (2018) Cognitive features and surgical outcome of patients with long-term epilepsy-associated tumors (LEATs) within the temporal lobe. Epilepsy Behav 88:25–32. https://doi.org/10.1016/j.yebeh.2018.08.028
Funding
This research was supported by the Core Research for Evolutional Science and Technology (CREST) of Japanese Science and Technology Agency (JPMJCR 20F1, JPMJCR 18A5); Health and Labor Science research grants for rare and intractable diseases from the Ministry of Health, Labor and Welfare, Japan (H29-nanchitou-ippan-010, 20FC1039); and Japan Society for the Promotion of Science (JSPS) KAKENHI (21K09160, 20H00235).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Kazuki Nomura wrote the first draft of the manuscript. Mari Tada and Akiyoshi Kakita made the pathological diagnosis. All the authors commented on previous versions and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All information is de-identified, including the photograph and radiological images. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Informed consent
The patient has consented to submission of this case report to the journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Neurosurgery—Epilepsy
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nomura, K., Suzuki, H., Iimura, Y. et al. Epilepsy surgery without lipoma removal for temporal lobe epilepsy associated with lipoma in the Sylvian fissure. Acta Neurochir 165, 265–269 (2023). https://doi.org/10.1007/s00701-022-05330-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05330-7