Abstract
Background
Schizencephaly is a rare congenital cerebral malformation characterized by clefts in the cerebral cortex. The traction effect and splitting of leptomeninges caused by schizencephaly potentially formed an arachnoid cyst.
Case presentation
We present a case of an 18-year-old male with history of several episodes of complex partial seizure over the last 2 months, with an electroencephalogram signified an epileptic form in the right temporal lobe. Brain magnetic-resonance imaging revealed an arachnoid cyst in the right temporal fossa and a grey matter-lined right temporal cleft consistent with open-lip schizencephaly. To the best of authors’ knowledge, only two previous similar cases have been reported in 1997. Our patient underwent cystoperitoneal shunt and was given double anti-epileptic agents, which successfully resolved and prevented the seizure episode during further follow-up.
Conclusion
New onset of seizure-associated schizencephaly in adult are very rare. In this case, space-occupying lesions effects from the enlarged arachnoid cysts caused seizure, which resolved after the cystoperitoneal shunt and double anti-epileptic agents.
Similar content being viewed by others
Background
Schizencephaly is a rare congenital cerebral malformation characterized by clefts in the cerebral cortex, unilaterally or bilaterally, that connects the ependymal surface of the lateral ventricle and the pia mater [1, 2]. The global incidence is only about 1.5 in 1,000,000 live births, with one in 1650 children case presents with epilepsy, which is very rare in adults [1, 2]. Schizencephaly classified into three: (1) Type-1/trans-mantle, the trans-mantle column of abnormal gray matter (GM), with no cerebrospinal fluid (CSF)-containing cleft, (2) Type-2/closed-lip, adjacentabnormal lips of GM in the presence of CSF-containing cleft, (3) Type 3/open-lip, non-adjacent abnormal lips of GM in the presence of CSF-containing cleft [3].
The etiopathogenesis is not clearly understood. It is usually accompanied by other intracranial anomalies (septo-optic dysplasia, GM-heterotopia, absence of septum pellucidum, and corpus callosum dysgenesis) [4]. However, there are several possible contributing risk factors such as young maternal-age, teratogenic agents exposure, attempted abortion, fetal hypoxia, amniocentesis, chorionic villus sampling, prenatal cytomegalovirus infection, genetic mutation, and fetal stroke/intracranial hemorrhage [5,6,7].
To the best of authors’ knowledge, there have been a total of two reported cases (1997) of the coexistence of schizencephaly and arachnoid cyst. The formation of arachnoid cysts is said to be formed due to the traction effect and splitting of leptomeninges by schizencephaly [8]. We report the findings of arachnoid cyst associated with open-lip schizencephaly presenting with epilepsy in 18-year-old male which resolved after surgical and pharmacological treatment.
Case presentation
An 18-year-old male patient was referred to our hospital of complex partial seizure over the last 2 months. His first episode was an absence seizure that lasted several seconds. Two weeks prior, he fell and his right-head colliding on the ground. He did not have any medical examination afterward. The second episode was a left-arm focal clonic seizure. The third was a tonic–clonic seizure with convulsions of the left-arm that lasted 10 min and was followed by a loss of consciousness period and retrograde amnesia. He never had any symptoms of nausea or headache. He and his family members have never experienced these symptoms before. However, his father has had a history of febrile seizures and an absence of folic acid supplementation in his mother during pregnancy. He is proportionally athletic, with no history of post-natal complication or global delay in developmental milestones.
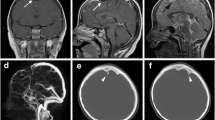
Physical and neurological examination revealed no abnormalities. During neurocognitive testing, has some degree of limitations in the mathematical and language sections on the Montreal Cognitive Assessment (MoCA) scoring with a total score of 22, interpreted as mild-cognitive impairment. Brain magnetic resonance imaging (MRI) with contrast showed an extra axial cystic lesion, non-enhanced-cerebrospinal fluid (CSF) attenuation in right temporal fossa, measuring ± 8.17 × 3.46 × 6.27 cm (antero-posterior × transverse × cranio-caudal) that caused a mass effect of adjacent brain parenchymal and mild leftward subfalcine herniation (Fig. 1).
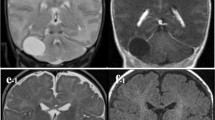
MRI also revealed GM-lined right temporal cleft extending from the right lateral ventricle to the cortex and filled with CSF. These findings are keeping with an arachnoid cyst and open-lip schizencephaly (Fig. 2). Electroencephalography (EEG) signified an epileptic form in the right temporal lobe. A cystoperitoneal shunt was performed (Fig. 3), and 750 mg levepiracetam was given twice daily. Five days later, the patient experienced right side facial focal seizure. One month later, he experienced absence seizure and he was given 1000 mg Levetiracetam twice daily and 2000 mg Sodium Divalproex once daily.
Discussion
Arachnoid cysts rarely cause complaints and are usually not explicitly treated; otherwise, open-lip schizencephaly often causes complaints [8]. Arachnoid cysts, an intracranial CSF-filled lesion lined with arachnoid membranes, only make up 1% of all intracranial space-occupying lesions (SOL) that are usually asymptomatic and often an incidental finding [9]. This case showed a schizencephaly caused a relatively large size of the arachnoid cyst and would continue to compress the surrounding structure, which led to episodes of seizures. Although the etiology is still poorly understood, the plausible risk factor, in this case, is the absence of consumption of folic acid during pregnancy. Similar findings have only been reported in two cases before, with the co-existence of arachnoid cyst caused by traction effect and splitting of the leptomeninges because of deep cortical infolding of the schizencephalic cleft [8].
The present of new-onset seizures in adults without a clear cause, other symptoms, and normal findings in physical and general neurological examinations make the need for advanced investigation to confirm neuroanatomical abnormalities. MRI provides excellent detailed images of anatomic changes, GM visualization, and to differentiate the fluid-associated central nervous system abnormalities. In this case of schizencephalic, each of the MR sequences helps show its different characteristics. T1-weighted (T1W) sequence shows cortex detail of schizencephaly. T2-weighted (T2W) sequences in the sagittal plane are used to evaluate polymicrogyria. Fluid-attenuated inversion recovery (FLAIR) sequence is precious to differentiate focal cortical dysplasia; and heterotopic grey matter, which is seen as hyperintense. In the case of an arachnoid cyst, MRI is also the best modality to illustrate the cyst’s anatomic location, size, surrounding structures and to differentiate other cause of cyst. The classic arachnoid cyst has no identifiable internal architecture and does not enhance [10, 11].
Management of schizencephaly usually prioritizes supportive and rehabilitative management. However, surgery is generally performed if hydrocephalus or raised intracranial pressure is present [12]. In the case of symptomatic arachnoid cyst that compresses surrounding structure, surgery should be performed. Surgical excision of the arachnoid cyst’ wall performed when the lesion is readily amendable, also, surgical/endoscopic fenestration can also be considered [9, 13].
A cystoperitoneal shunt is rarely used due to the complications that can arise with long-term shunt placement; however, it is needed as an emergency choice when significant hydrocephalus or mass effect is present [9, 13]. As in this case, cystoperitoneal shunt was performed to reduce the midline shift and the size of the arachnoid cyst. Surgical excision was not performed because the schizencephaly would still raise the intracranial pressure. After surgery, anti-epileptic medications were initiated, and the patient did not experience any episodes of seizures during a month of follow-up. However, further observation must be done periodically.
Conclusions
New onset of seizure-associated schizencephalyin adult are very rare. Epilepsy in open-lip schizencephaly of this case resulting from space-occupying lesions (SOL) effects of enlarged arachnoid cysts. The episode of seizure was successfully resolved after the cystoperitoneal shunt that reduced the arachnoid cyst size and double anti-epileptic agents.
Availability of data and materials
Essential data supporting the findings of this study are available within the article. Further data are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Abbreviations
- AP:
-
Anterior–posterior
- CC:
-
Cranio-caudal
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- EEG:
-
Electroencephalography
- FLAIR:
-
Fluid-attenuated inversion recovery
- GM:
-
Gray matter
- MoCA:
-
Montreal Cognitive Assessment
- MRI:
-
Magnetic-resonance imaging
- SOL:
-
Space-occupying lesions
- T1W:
-
T1-weighted
- T2W:
-
T2-weighted
- TR:
-
Transverse
References
Hung PC, Wang HS, Chou ML, Lin KL, Hsieh MY, Chou IJ, et al. Schizencephaly in children: a single medical center retrospective study. Pediatr Neonatol. 2018;59:573–80.
Kamble V, Lahoti AM, Dhok A, Taori A, Pajnigara N. A rare case of schizencephaly in an adult with late presentation. J Fam Med Prim Care. 2017;6:450–2.
Griffiths PD. Schizencephaly revisited. Neuroradiology. 2018;60:945–60.
Doğan E. The evaluation of unilateral closed-lip schizencephaly on complex MRI sequences. Rom J Neurol. 2021;20:250–4.
Gonzalez JC, Singhapakdi K, Martino AM, Rimawi BH, Bhat R. Unilateral open-lip schizencephaly with tonsillar herniation in a preterm infant. J Pediatr Neurosci. 2019;14:225–7.
Dies KA, Bodell A, Hisama FM, Guo CY, Barry B, Chang BS, et al. Schizencephaly: association with young maternal age, alcohol use, and lack of prenatal care. J Child Neurol. 2013;28:198–203.
Harada T, Uegaki T, Arata K, Tsunetou T, Taniguchi F, Harada T. Schizencephaly and porencephaly due to fetal intracranial hemorrhage: a report of two cases. Yonago Acta Med. 2018;60:241–5.
Sener RN. Coexistence of schizencephaly and middle cranial fossa arachnoid cyst: a report of two patients. Eur Radiol. 1997;7:409–11.
Mustansir F, Bashir S, Darbar A. Management of arachnoid cysts: a comprehensive review. Cureus. 2018;10: e2458.
Jafrani R, Raskin JS, Kaufman A, Lam S. Intracranial arachnoid cysts: pediatric neurosurgery update. Surg Neurol Int. 2019;10:15.
Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006;239:650–64.
Halabuda A, Klasa L, Kwiatkowski S, Wyrobek L, Milczarek O, Gergont A. Schizencephaly-diagnostics and clinical dilemmas. Childs Nerv Syst. 2015;31:551–6.
Al-Holou WN, Terman S, Kilburg C, Garton HJL, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg. 2013;118:222–31.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
PH, CN, and RS contributed to writing and interpreting the data. C, A, and R were the responsible doctor in providing the clinical data and patient management. The manuscript submitted has been read and approved by all authors, the requirements for authorship have been met, and authors believe that the manuscript represents honest work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval is not applicable for the case report. All written informed consent for medical procedures and patient’s medical information study was obtained from the patient to publish this case report and accompanying images. All ethical principles for medical research studies established by our hospital have been followed.
Consent for publication
All written informed consent for medical procedures and patient’s medical information study was obtained from the patient to publish this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hapsari, P., Celia, Nagpal, C. et al. Epilepsy-associated open-lip schizencephaly with arachnoid cyst: a rare case report. Egypt J Neurol Psychiatry Neurosurg 59, 23 (2023). https://doi.org/10.1186/s41983-023-00625-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00625-7