Abstract
Purpose
Anaemia is common in patients presenting with aneurysmal subarachnoid (aSAH) and intracerebral haemorrhage (ICH). In surgical patients, anaemia was identified as an idenpendent risk factor for postoperative mortality, prolonged hospital length of stay (LOS) and increased risk of red blood cell (RBC) transfusion. This multicentre cohort observation study describes the incidence and effects of preoperative anaemia in this critical patient collective for a 10-year period.
Methods
This multicentre observational study included adult in-hospital surgical patients diagnosed with aSAH or ICH of 21 German hospitals (discharged from 1 January 2010 to 30 September 2020). Descriptive, univariate and multivariate analyses were performed to investigate the incidence and association of preoperative anaemia with RBC transfusion, in-hospital mortality and postoperative complications in patients with aSAH and ICH.
Results
A total of n = 9081 patients were analysed (aSAH n = 5008; ICH n = 4073). Preoperative anaemia was present at 28.3% in aSAH and 40.9% in ICH. RBC transfusion rates were 29.9% in aSAH and 29.3% in ICH. Multivariate analysis revealed that preoperative anaemia is associated with a higher risk for RBC transfusion (OR = 3.25 in aSAH, OR = 4.16 in ICH, p < 0.001), for in-hospital mortality (OR = 1.48 in aSAH, OR = 1.53 in ICH, p < 0.001) and for several postoperative complications.
Conclusions
Preoperative anaemia is associated with increased RBC transfusion rates, in-hospital mortality and postoperative complications in patients with aSAH and ICH.
Trial registration
ClinicalTrials.gov, NCT02147795, https://clinicaltrials.gov/ct2/show/NCT02147795
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
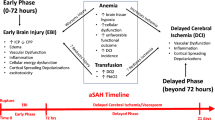
Anaemia is an independent risk factor for postoperative complications, mortality, prolonged hospital length of stay (LOS) and increased risk of red blood cell (RBC) transfusion [24]. The prevalence of anaemia is reported to be 22.8% globally and 26.5-31.5% in patients undergoing surgery [2, 10]. Preoperative anaemia was reported for 5.5% of patients suffering from aneurysmal subarachnoid haemorrhage (aSAH) [8] and for 24.1–25.8% of patients suffering from intracerebral haemorrhage (ICH) [16, 17]. For patients with ICH, anaemia has also been shown to be an independent predictor for unfavourable long-term outcomes a decade ago [17]. Kumar et al. demonstrated that anaemia is common in acute ICH patients and that its presence on admission is an independent predictor of increased ICH volume; contrary to pathophysiological considerations, however, in 2009, they could not demonstrate an effect on increased mortality [16].
The treatment of anaemia in emergency situations usually involves the administration of allogeneic blood products. The administration of RBC transfusions is known to be associated with multiple risks, such as transfusion-related lung injury, haemolytic reactions and transmission of infectious diseases [12]. RBC transfusions in patients undergoing cranial surgery are also associated with a prolonged LOS, more postoperative complications, a 30-day return to the operating theatre and an increased 30-day mortality rate [4]. In patients with aSAH, RBC transfusions have been shown to result in increased mortality and general worse clinical outcomes [31].
This multicentre cohort study analyses the incidence of preoperative anaemia and its association with RBC transfusion requirements, hospital length of stay (LOS), in-hospital mortality and clinically relevant outcomes in patients with aSAH and ICH.
Methods
Study design and objectives
The current study is a subanalysis of the ongoing prospective multicentre observational study ‘Safety and effectiveness of a Patient Blood Management (PBM) programme in surgical patients’ (ClinicalTrials.gov, NCT02147795) [22]. The period analysed covered 1 January 2010 to 30 September 2020. Data from 23 hospitals was screened. The study was approved by the Ethics Committee of the University Hospital Frankfurt, Goethe University (first vote ref. 380/12 from 10 January 2013, amendments from 17 June 2013 to 1 June 2016, second vote ref. 318/17 from 30 November 2017) who waived the requirement for informed patient’s consent. In addition, the local ethics committee of each participating centre followed this vote and likewise waived the requirement for informed patient’s consent.
The primary objective of the study was to assess the prevalence of preoperative anaemia and its association with RBC transfusion in aSAH or ICH patients. The secondary objective was to investigate the association of potential risk factors (such as preoperative anaemia, RBC transfusions and other factors related with the type of neurosurgical intervention, additional diagnoses and patient characteristics) with common clinical outcomes (including mortality, typical postoperative complications and LOS) in patients with aSAH and ICH (Online Resource 1).
Patient enrolment and inclusion criteria
The underlying PBM database was contained by design adult (≥ 18 years) in-hospital patients, who underwent surgery or a procedure (classified according to the Operation and Procedure Classification System (OPS) code (Online Resource 1)) during their hospital stay. Patients from the PBM database with a diagnosis of aSAH or ICH, defined by the International Classification of Disease (ICD-10) codes and discharged from hospital within the time period from 1 January 2010 to 30 September 2020, were included (Online Resource 1 and 2). The exclusion criteria were the diagnoses of additional traumatic SAH and intracranial neoplasm (Online Resource 1). Patients were assigned to either the aSAH (patients diagnosed with aSAH with/without additional ICH) or the ICH group (patients diagnosed with ICH only) (Fig. 1). This classification was chosen because an additional ICH can occur after the aetiological event of an aSAH, even though the patients were originally diagnosed only with aSAH. In the group of patients diagnosed with ICH only, ICH was the primary diagnosis and cause of hospitalisation.
Definitions
Anaemia was defined according to the WHO definition: anaemia Hb < 12 g/dl (7.45 mmol/l) (female) and Hb < 13 g/dl (8.07 mmol/l) (male); mild anaemia in female: Hb 11-12 g/dl (6.83-7.45 mmol/l); mild anaemia in male: Hb 11-13 g/dl (6.83-8.07 mmol/l); moderate anaemia in female and male: Hb 8-11 g/dl (4.97-6.83 mmol/l); severe anaemia in female and male Hb < 8 g/dl (< 4.97 mmol/l) [27].
The preoperative anaemia status was based on the first available preoperative Hb value, and the postoperative anaemia status was based on the last available Hb value before hospital discharge. Diagnostic criteria were defined by the relevant ICD-10 codes (Online Resource S1). Vasospasm was defined by ICD code I67.80. Interventions were defined by the relevant Operation and Procedure Classification (OPS) codes (Online Resource S1). Mortality was defined by the discharge code. Hospital LOS was defined by the given admission and discharge dates.
Data collection
The underlying data source was an anonymous routine data from hospital information systems (e.g. Agfa Orbis, Nexus, iMedOne, SAP) and additional data from individual blood bank and pharmacy software systems of the corresponding hospitals participating in the epidemiological research and quality management study of the German Patient Blood Management Network [22]. The data transferred to the PBM Network Coordination Centre did not contain any personal information. A data protection vote from the Hessian data protection officer was obtained (ref: 43.60; 60.01.21-ga from 24 October 2018). The biostatistician in charge subsequently evaluated the data for completeness and correctness and extracted the cases that fulfilled the inclusion criteria for this study before performing the final analysis.
Statistical analysis
Descriptive analysis was used to determine patient characteristics, the prevalence of pre- and postoperative anaemia, RBC transfusions, surgical interventions and postoperative outcomes. The results of the descriptive analysis are presented as means (± standard errors), medians (with first and third quartiles) and rates (with 95% CI).
Multivariate mixed effect regression analysis was performed to identify independent predictors of RBC transfusion and various postoperative outcomes. The multivariate mixed effect regression models included the hospitals as random effects (to account for hospital individual effects) and other potentially relevant factors (such as age, gender, surgical interventions, preoperative anaemia, RBC consumption and vasospasm) as fixed effects.
Univariate non-parametric analysis (chi-square tests for binary endpoints and Wilcoxon-Mann-Whitney tests for continuous endpoints) was performed a priori to assess the correlation of the individual factors where appropriate. To account for the heterogeneity of the aSAH and ICH groups, all analyses (univariate and multivariate) were performed separately by group. All analyses were performed using the free software R (version 3.6.3).
Results
A total of n = 1,325,438 patients from 23 hospitals were screened. Two hospitals in the network did not treat patients with neurosurgical diagnoses. Overall, n = 9081 eligible patients from 21 hospitals were included and analysed in this study. The aSAH group included n = 5008 patients and the ICH group included n = 4073 patients (Fig. 1). The incidence of eligible cases within the entire database of n = 1,325,438 was 0.4% for aSAH and 0.3% for ICH. Most patients received a neurosurgical OPS (84.9% in aSAH and 76.9% in ICH). The remaining OPS are distributed across several specialties (visceral and endocrine surgery accounts for the highest proportion with 5.0%, followed by 3.5% with otorhinolaryngology). The distribution of other surgical OPS can be found in Online Resource 2). Demographic and intervention data are shown in Table 1.
Anaemia
The median preoperative Hb level was 13.2 g/dl in aSAH patients and 12.8 g/dl in ICH patients. Severe, moderate and mild preoperative anaemia was present in aSAH patients at rates of 1.0%, 10.7% and 16.6%, respectively and in ICH patients at rates of 2.7%, 17.6% and 20.6%, respectively (Table 1).
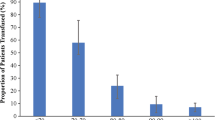
Descriptive and univariate analysis for postoperative outcomes according to preoperative anaemia are listed for both pathologies in Tables 1 and 2 and Online Resource Tables 3 and 4. Mortality was significantly higher in the presence of preoperative anaemia (22.2% versus 13.3%, p < 0.001 in aSAH and 31.5% versus 17.9%, p < 0.001 in ICH) (Table 2). Figure 2 demonstrates that an increase in the preoperative Hb values corresponds to a decrease in the mortality rate.
Descriptive and univariate analysis revealed that preoperative anaemia resulted in significantly higher numbers of RBC units transfused, LOS, postoperative anaemia, renal failure and sepsis both for aSAH und ICH patients (Tables 1, 2, and 3, Online Resource Tables 3 and 4). Vasospasm was significantly lower in the presence of preoperative anaemia (9.3% versus 12.4%, p = 0.004) in aSAH patients (Table 1). Multivariate analysis showed that preoperative anaemia was an independent risk factor for increased RBC transfusion in both patients with aSAH (p < 0.001; OR = 3.25) and ICH (p < 0.001; OR = 4.16) (Tables 4 and 5). Multivariate analysis indicated preoperative anaemia was an independent risk factor for mortality (OR = 1.48 in aSAH patients, OR = 1.53 in ICH patients, both p < 0.001), transfused RBC units (p < 0.001) and postoperative anaemia (OR = 6.18 in aSAH patients, OR = 7.11 in ICH patients, p < 0.001). In aSAH patients, moreover, preoperative anaemia increased the risk for renal failure (OR = 1.61, p = 0.002) and LOS (+ 1.6 days, p = 0.03). Preoperative anaemia was an independent factor for decreased LOS in ICH (−2.5 days, p = 0.006). Furthermore, preoperative anaemia was an independent factor for decreased ischemic stroke (OR = 0.78, p = 0.005 in aSAH and OR = 0.82, p = 0.05 in ICH), pneumonia (OR = 0.78 in ICH, p = 0.008), pulmonary embolism (OR = 0.60, p = 0.02 in aSAH) and vasospasm (OR = 0.70, p = 0.01 in aSAH) (Tables 5 and 6).
RBC transfusion
RBC transfusion rates were higher in the presence of preoperative anaemia in both the aSAH group (45.8% vs 24.9%, p < 0.001) and the ICH group (45.0% vs 18.8%, p < 0.001), (Table 3 and Online Resource Tables 3 and 4). Figure 3 demonstrates that a constant increase in the preoperative Hb values corresponds to a constant decrease in the RBC transfusion rate. Preoperative anaemic patients were significantly more likely to receive RBC transfusions than non-anaemic patients (24.9% vs. 45.8%, p < 0.001 in aSAH and 18.8% vs. 45.0%, p < 0.001 in ICH) (Table 3 and Online Resource Tables 3 and 4). In the additional descriptive analysis, transfusion rates for RBC, plasma and clotting products were higher when haemorrhagic diatheses due to coumarins, heparins and novel oral anticoagulants (NOACs), as well as factor XIII and factor VIII deficiency, were present (Table 3 and 4). Mortality rates were higher when more RBC units were required (Table 2).
Multivariate analysis revealed that RBC transfusion was an independent (all p < 0.001) risk factor for increased mortality (OR = 3.59 in ICH, OR = 2.30 in aSAH), LOS (+ 17.7 days in ICH, + 13.7 days in aSAH), ischaemic stroke, renal failure, sepsis, pneumonia and pulmonary embolism in aSAH and ICH patients and for vasospasm (OR = 2.47) in aSAH patients (Table 5 and 6).
Interventions
In the univariate and descriptive analysis, RBC transfusion rates were significantly (p < 0.001) higher in the presence of interventions (Table 2 and Online Resource Tables 3 and 4). In the multivariate analysis, clipping was an independent factor for significantly lesser RBC units transfused (−679 units/1000 patients, p = 0.035) in aSAH patients. Coiling (OR = 1.63, p < 0.001) and craniotomy (OR = 2.30, p < 0.001) were independent risk factors for significantly higher RBC transfusion rates in aSAH patients. Craniotomy was independently associated with significantly higher RBC transfusion rates (OR = 1.78, p < 0.001 in ICH and OR = 2.30, p < 0.001 in aSAH) and RBC units transfused (+ 1263 units/1000 patients, p < 0.001 in ICH) (Table 5 and 6)
Vasospasm
The proportion of preoperative anaemia was significantly lower (p = 0.004) in the vasospasm (22.8%) group than in the non-vasospasm group (29.0%). The RBC transfusion rate was significantly higher (p < 0.001) in the vasospasm (40.9%) than in the non-vasospasm group (28.1%) (Online Resource 5). In the multivariate analysis, vasospasm was an independent risk factor for RBC transfusion (OR = 2.13, p < 0.001), postoperative anaemia (OR = 1.67, p = 0.004), prolonged LOS (+ 6.1 days, p < 0.001), pneumonia (OR = 1.45, p < 0.001) and ischemic stroke (OR = 1.45, p = 0.001) (Tables 3 and 4).
Discussion
The data revealed in both aSAH and ICH patients that preoperative anaemia is associated with a higher RBC transfusion rate, increased postoperative in-hospital mortality and increased complication rates. These findings align with the study results for other patient cohorts. Thus, in neurosurgical patients, preoperative anaemia has been shown to be an independent risk factor for postoperative mortality and increased risk of RBC transfusion [24]. Anaemia is common in aSAH patients [8, 14, 30, 33] and in ICH patients [16, 17]. In this study, the prevalence of preoperative anaemia in both groups (aSAH 28.3% and ICH 40.9%) was higher than described in previous publications (aSAH 5.5% and ICH 24.1-25.8%) [8, 16, 17]. One explanation for this could be that the database only includes cohorts of patients who underwent surgery or other interventions (e.g., coiling) during a hospital stay, so that selection bias cannot be ruled out.
The physiological and pathophysiological impact of anaemia in patients with aSAH and ICH is multifactorial. The supply of oxygen to the brain depends on several variables. Cerebral oxygen availability (DO2) is the product of cerebral blood flow (CBF) and arterial oxygen content (CaO2): DO2 = CBF × CaO2 [19]. The oxygen content (CaO2) itself is represented by the formula CaO2 = (1.31 × Hb × SaO2 × 0.01) + (0.0225 × PaO2) and thus depends on Hb levels, arterial oxygen saturation (SaO2) and arterial oxygen pressure (PaO2) [7]. The formula demonstrates that apart from an increase in SaO2, the most significant factor for optimising the DO2 to the target cell is the Hb value; thus, the need arises to consider ways of increasing the Hb value through various measures (such as anaemia management or transfusion in an emergency). In a healthy brain, a progressive decrease in Hb is compensated for by vasodilation, resulting in increased CBF and a constant cerebral oxygen supply DO2. When Hb falls below 5-6 g/dL, DO2 decreases and no further vasodilation can occur and maximum CBF levels are reached [19]. We observed that the vasospasm rate was significantly lower with preoperative anaemia. It is possible, that the Hb value influences only patients’ outcomes after cerebral vasospasm and not the probability of a cerebral vasospasm event itself. The multivariate analysis also revealed a significant association of RBC transfusion (OR = 2.47, p < 0.001) with vasospasm. This finding underlines the need for risk assessment prior to transfusion and additional prospective studies on this topic. Scholars have long debated whether elevating the haemoglobin levels in SAH patients with vasospasm and thus avoiding anaemia is beneficial [15, 18, 29]. In general, based on CONSCIOUS-3, the role of vasospasm on delayed cerebral ischemia should be considered with caution, where clazosentan was shown to significantly reduce postaSAH vasospasm, but neither dose improved outcome [20]. Further studies are needed to prove the potential beneficial effects of RBC transfusion on anaemic aSAH patients suffering from cerebral vasospasm. In the field of critical care, there is a growing evidence that strict transfusion limits remain best practice for the vast majority of cases, due to limited adverse effects, comparable or better clinical outcomes and economic aspects [28]. Thus, a restrictive threshold for RBC transfusions (Hb < 7 g/dl) is still recommended in both critically ill and clinically stable ICU patients [23]. Similar pathophysiological considerations are known in patients with acute myocardial infarction, as a recent study demonstrated that a restrictive transfusion strategy resulted in less major adverse cardiac events after 30 days (11.0% in the restrictive and 14.0% in the liberal group) [6]. In the retrospective study by English et al., only 20% of patients with aSAH received RBC transfusions, mostly in the presence of significant anaemia (Hb < 8 g/dl), and this was not associated with worse outcomes [8]. However, Dhar et al. demonstrated that RBC transfusion in aSAH patients improved cerebral oxygenation both globally and particularly in the vulnerable brain regions and thus may potentially minimise the risk for delayed cerebral ischaemia. The study analysed the outcomes over a wide range of haemoglobin levels and suggests that restrictive transfusion practice may not be appropriate in this vulnerable population [5]. Naidech et al. demonstrated no difference in outcomes in SAH for Hb 10.0 versus 11.5 g/dL. Here, however, the difference between the groups is rather minor and well-above general limits for transfusions [25]. The answer to the question of the role of treatment of anaemia with red blood cell transfusion could be provided by the still ongoing SAHaRA trial [9]. In our analysis, mortality was increased considerably when more transfusions were given, which is also in line with the results from Ceanga et al. [3].
Although preoperative diagnosis and treatment of anaemia can only be implemented to a limited extent in acute situations of ICH and aSAH, the present data underlines primarily the importance of general anaemia vigilance and treatment (as ICH and aSAH occur sudden and without time frame for treatment), and secondarily, anaemia treatment becomes important in the context of peri-/postoperative care. To identify and manage anaemia at an early stage, a multimodal therapy using patient blood management (PBM) has been developed. PBM is an evidence-based, patient-centred and multidisciplinary approach to minimise anaemia-associated risks, unnecessary blood loss and transfusions in patients undergoing surgery [1]. For this purpose, measures have been implemented to reduce preoperative anaemia, minimise iatrogenic blood loss and optimise patient-specific anaemia tolerance [11]. If iron deficiency is identified in the absence of infection, iron supplementation and erythropoiesis-stimulating agents can be considered [13]. Measures to reduce intraoperative blood loss and optimise coagulopathy should be implemented. This includes the following measures (also in neurosurgery): Treatment of coagulopathy should be based on a fixed algorithm. The content of the coagulation algorithm should be the maintenance of basic conditions for haemostasis (body temperature > 36 °C, ionised calcium > 1.1 mmol/ L, pH > 7.2) or point-of-care diagnostics. The prevalence of bleeding due to anticoagulation was low in our analysis but point of care technology provides information on coagulation dysfunction and the use of anticoagulation, including NOACs. The use of an antifibrinolytic is safe and recommended [32]. Blood sample collections should be reduced to the absolute necessary numbers, blood sample collection tubes should draw as little blood volume as possible, and return systems for blood sample collections should be established. Washed cell salvage—the collection, washing and retransfusion of a patient’s own wound blood—can help to reduce the need for blood from other sources [21, 26].
Limitations
Although studies with routine data have several important advantages over traditional clinical trials (such as a larger number of cases with fewer personnel, time and cost requirements) and are therefore becoming increasingly popular as an alternative in the age of advancing digitalisation, they naturally also have some disadvantages. This study is based on routine data of hospital information systems. Data quantity and quality varied between hospitals. In addition, routine data may have some other limitations, in general, such as missing data or incorrect coding techniques. Since ICD and OPS codes are billing-related, they may be biased.
Furthermore, there is no information on the exact time of occurrence and duration of complications and perioperative interventions (including blood transfusions), so an association does not necessarily indicate causality, nor is it possible to show the direction of causality. For this reason, we report associations rather than causalities of the factors.
Missing information on neurological status, resuscitation and intercurrent diseases cannot be obtained from the register, so that a limitation in the analysis of associations with anaemia and transfusion is possible here. The analysis could not consider the influence of a potential — and already locally available — anaemia therapy. The Hunt and Hess scale for aSAH, which measures the severity of the aSAH, is not documented in ICD-10 codes; therefore, a severity-adapted evaluation was not possible. In neurosurgical therapy, patients are often transferred to a rehabilitation intensive care unit or back to the referring intensive care unit shortly after treatment; this leads to a possible bias in endpoints (e.g., especially for LOS). This is a retrospective analysis of prospectively collected registry data; limitations of a retrospective analysis cannot be avoided.
Conclusions
Preoperative anaemia is associated with increased RBC transfusion rates, in-hospital mortality, and postoperative complications in patients with aSAH and ICH. Prospective multicentre studies with tailored data on the therapy of anaemia, the optimal haemoglobin value and transfusion strategy, both for aSAH and ICH patients, are urgently needed.
Abbreviations
- aSAH:
-
aneurysmal subarachnoid haemorrhage
- CI:
-
confidence interval
- Hb:
-
haemoglobin
- ICD:
-
International Classification of Disease
- ICH:
-
intracerebral haemorrhage
- IQR:
-
interquartile range
- LOS:
-
Length of in-hospital stay
- NOAC:
-
novel oral anticoagulants
- OPS:
-
Operation and Procedure Classification
- OR:
-
odds ratio
- RBC:
-
red blood cells
- SE:
-
standard error
References
Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Fullenbach C, Freedman J, Waters JH, Farmer S, Leahy MF, Zacharowski K, Meybohm P, Choorapoikayil S (2019) Multimodal patient blood management program based on a three-pillar strategy: a systematic review and meta-analysis. Ann Surg 269:794–804. https://doi.org/10.1097/SLA.0000000000003095
Baron DM, Hochrieser H, Posch M, Metnitz B, Rhodes A, Moreno RP, Pearse RM, Metnitz P, European Surgical Outcomes Study group for Trials Groups of European Society of Intensive Care M, European Society of A (2014) Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 113:416–423. https://doi.org/10.1093/bja/aeu098
Ceanga AI, Ceanga M, Eveslage M, Herrmann E, Fischer D, Haferkamp A, Wittmann M, Muller S, Van Aken H, Steinbicker AU (2018) Preoperative anemia and extensive transfusion during stay-in-hospital are critical for patient`s mortality: a retrospective multicenter cohort study of oncological patients undergoing radical cystectomy. Transfus Apher Sci 57:739–745. https://doi.org/10.1016/j.transci.2018.08.003
Cohen JA, Alan N, Seicean A, Weil RJ (2017) Risk associated with perioperative red blood cell transfusion in cranial surgery. Neurosurg Rev 40:633–642. https://doi.org/10.1007/s10143-017-0819-y
Dhar R, Zazulia AR, Derdeyn CP, Diringer MN (2017) RBC Transfusion improves cerebral oxygen delivery in subarachnoid hemorrhage. Crit Care Med 45:653–659. https://doi.org/10.1097/CCM.0000000000002266
Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, Lemesle G, Cachanado M, Durand-Zaleski I, Arnaiz JA, Martinez-Selles M, Silvain J, Ariza-Sole A, Ferrari E, Calvo G, Danchin N, Avendano-Sola C, Frenkiel J, Rousseau A, Vicaut E, Simon T, Steg PG, Investigators R (2021) Effect of a restrictive vs liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: the REALITY randomized clinical trial. JAMA 325:552–560. https://doi.org/10.1001/jama.2021.0135
Dunn J-O, Mythen M, Grocott M (2016) Physiology of oxygen transport. Bja. Education 16:341–348
English SW, Chasse M, Turgeon AF, Lauzier F, Griesdale D, Garland A, Fergusson D, Zarychanski R, van Walraven C, Montroy K, Ziegler J, Dupont-Chouinard R, Carignan R, Dhaliwal A, Mallick R, Sinclair J, Boutin A, Pagliarello G, Tinmouth A, McIntyre L, Canadian Critical Care Trials G (2018) Anemia prevalence and incidence and red blood cell transfusion practices in aneurysmal subarachnoid hemorrhage: results of a multicenter cohort study. Crit Care 22:169. https://doi.org/10.1186/s13054-018-2089-7
English SW, Fergusson D, Chasse M, Turgeon AF, Lauzier F, Griesdale D, Algird A, Kramer A, Tinmouth A, Lum C, Sinclair J, Marshall S, Dowlatshahi D, Boutin A, Pagliarello G, McIntyre LA, Canadian Critical Care Trials G (2016) Aneurysmal subarachnoid hemorrhage-red blood cell transfusion and outcome (SAHaRA): a pilot randomised controlled trial protocol. BMJ Open 6:e012623. https://doi.org/10.1136/bmjopen-2016-012623
Gardner W, Kassebaum N (2020) Global, regional, and national prevalence of anemia and its causes in 204 countries and territories, 1990–2019. Current Developments in Nutrition 4:830–830. https://doi.org/10.1093/cdn/nzaa053_035
Goodnough LT, Shander A (2012) Patient blood management. Anesthesiology 116:1367–1376. https://doi.org/10.1097/ALN.0b013e318254d1a3
Hendrickson JE, Hillyer CD (2009) Noninfectious serious hazards of transfusion. Anesth Analg 108:759–769. https://doi.org/10.1213/ane.0b013e3181930a6e
Investigators I, Litton E, Baker S, Erber WN, Farmer S, Ferrier J, French C, Gummer J, Hawkins D, Higgins A, Hofmann A, De Keulenaer B, Mc Morrow J, Olynyk JK, Richards T, Towler S, Trengove R, Webb S, Australian, New Zealand Intensive Care Society Clinical Trials G (2016) Intravenous iron or placebo for anaemia in intensive care: the IRONMAN multicentre randomized blinded trial: a randomized trial of IV iron in critical illness. Intensive Care Med 42:1715–1722. https://doi.org/10.1007/s00134-016-4465-6
Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP (2008) Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. Crit Care Med 36:2070–2075. https://doi.org/10.1097/CCM.0b013e31817c1095
Kumar MA, Levine J, Faerber J, Elliott JP, Winn HR, Doerfler S, Le Roux P (2017) The effects of red blood cell transfusion on functional outcome after aneurysmal subarachnoid hemorrhage. World Neurosurg 108:807–816. https://doi.org/10.1016/j.wneu.2017.09.038
Kumar MA, Rost NS, Snider RW, Chanderraj R, Greenberg SM, Smith EE, Rosand J (2009) Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med 37:1442–1447. https://doi.org/10.1097/CCM.0b013e31819ced3a
Kuramatsu JB, Gerner ST, Lucking H, Kloska SP, Schellinger PD, Kohrmann M, Huttner HB (2013) Anemia is an independent prognostic factor in intracerebral hemorrhage: an observational cohort study. Crit Care 17:R148. https://doi.org/10.1186/cc12827
Le Roux PD, Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H (2011) Anemia and transfusion after subarachnoid hemorrhage. Neurocrit Care 15:342–353. https://doi.org/10.1007/s12028-011-9582-z
Lelubre C, Bouzat P, Crippa IA, Taccone FS (2016) Anemia management after acute brain injury. Crit Care 20:152. https://doi.org/10.1186/s13054-016-1321-6
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Nowbakht P, Roux S, Kassell N (2012) Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 43:1463–1469. https://doi.org/10.1161/STROKEAHA.111.648980
Meybohm P, Choorapoikayil S, Wessels A, Herrmann E, Zacharowski K, Spahn DR (2016) Washed cell salvage in surgical patients: a review and meta-analysis of prospective randomized trials under PRISMA. Medicine (Baltimore) 95:e4490. https://doi.org/10.1097/MD.0000000000004490
Meybohm P, Fischer DP, Geisen C, Muller MM, Weber CF, Herrmann E, Steffen B, Seifried E, Zacharowski K, German PBMSCG (2014) Safety and effectiveness of a Patient Blood Management (PBM) program in surgical patients--the study design for a multi-centre prospective epidemiologic non-inferiority trial. BMC Health Serv Res 14:576. https://doi.org/10.1186/s12913-014-0576-3
Mueller MM, Van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, Carson JL, Cichutek K, De Buck E, Devine D, Fergusson D, Follea G, French C, Frey KP, Gammon R, Levy JH, Murphy MF, Ozier Y, Pavenski K et al (2019) Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA 321:983–997. https://doi.org/10.1001/jama.2019.0554
Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, Soweid A, Hoballah JJ, Taher AT, Jamali FR (2011) Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 378:1396–1407. https://doi.org/10.1016/S0140-6736(11)61381-0
Naidech AM, Shaibani A, Garg RK, Duran IM, Liebling SM, Bassin SL, Bendok BR, Bernstein RA, Batjer HH, Alberts MJ (2010) Prospective, randomized trial of higher goal hemoglobin after subarachnoid hemorrhage. Neurocrit Care 13:313–320. https://doi.org/10.1007/s12028-010-9424-4
Neef V, Vo L, Herrmann E, Triphaus C, Judd L, Winter A, Zacharowski K, Choorapoikayil S, Meybohm P (2021) The association between intraoperative cell salvage and red blood cell transfusion in cardiac surgery - an observational study in a patient blood management centre. Anaesthesiol Intensive Ther 53:1–9. https://doi.org/10.5114/ait.2021.103735
World Health Organization (2011) Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. No. WHO/NMH/NHD/MNM/11.1. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf
Rauer MJ, Neef V, Berra L (2021) Severe bleeding in the ICU. Curr Opin Anaesthesiol 34:530–536. https://doi.org/10.1097/ACO.0000000000001019
Rosenberg NF, Koht A, Naidech AM (2013) Anemia and transfusion after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 25:66–74. https://doi.org/10.1097/ANA.0b013e31826cfc1d
Sampson TR, Dhar R, Diringer MN (2010) Factors associated with the development of anemia after subarachnoid hemorrhage. Neurocrit Care 12:4–9. https://doi.org/10.1007/s12028-009-9273-1
Smith MJ, Le Roux PD, Elliott JP, Winn HR (2004) Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg 101:1–7. https://doi.org/10.3171/jns.2004.101.1.0001
Taeuber I, Weibel S, Herrmann E, Neef V, Schlesinger T, Kranke P, Messroghli L, Zacharowski K, Choorapoikayil S, Meybohm P (2021) Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg:e210884. https://doi.org/10.1001/jamasurg.2021.0884
Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA (2006) Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med 34(617-623):quiz 624. https://doi.org/10.1097/01.ccm.0000201903.46435.35
Acknowledgements
The German Patient Blood Management (PBM) Network with the associated registry was awarded the Lohfert Prize by the Lohfert Foundation in 2014, the German Prize for Patient Safety by Aktionsbündnis Patientensicherheit e.V. in 2016, the Humanitarian Award by the Patient Safety and Movement Foundation in 2016 and the MSD Health Prize by MSD SHARP & DOHME GMBH, Germany (3rd place) in 2018. We would like to express our sincere thanks for these honours.
We also wish to thank the IT staff of the participating centres for providing routine and additional data. In addition, we would like to thank the medical staff of each centre for their efforts in the practical implementation of PBM. We thank Dr. Christoph Füllenbach for his previous general support for the PBM project.
German PBM Network Collaborators
Klinikum Westmuensterland Bocholt: Olaf Baumhove (Department of Anaesthesiology)
University Hospital Bonn: Markus Velten, Maria Wittmann (Department of Anaesthesiology and Operative Intensive Care Medicine), Tim Lampmann, Erdem Güresir (Department of Neurosurgery)
DonauIsar Klinikum Deggendorf/Dingolfing/Landau: Diana Narita (Institute of Laboratory Diagnostics und Transfusion Medicine)
University Hospital Frankfurt am Main: Elisabeth Adam, Suma Choorapoikayil, Christoph Füllenbach, Lotta Hof, Sabine Isik, Martin Krämer, Holger Neb, Vanessa Neef, Florian Piekarski, Elke Schmitt, Kai Zacharowski (Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy), Thomas Walther, Thomas Holubec, Harald Keller (Department of Thoracic and Cardiovascular Surgery), Andreas Schnitzbauer, Wolf Otto Bechstein (Department of General and Visceral Surgery), Wojciech Derwich, Thomas Schmitz-Rixen (Department of Vascular and Endovascular Surgery), Bjoern Steffen, Hubertus Serve (Department of Haematooncology), Joerg Bojunga, Stefan Zeuzem (Department of Gastroenterology and Hepatology), Jürgen Konczalla, Volker Seifert (Department of Neurosurgery), Frederik Roos, Felix Chun (Department of Urology), Christoph Nau, Ingo Marzi (Department of Trauma, Hand and Reconstructive Surgery), Martin Leinung, Timo Stöver (Department of Otorhinolaryngology), Shahram Ghanaati, Robert Sader (Department of Oral Maxillofacial and Plastic Facial Surgery), Frank Louwen, Sven Becker (Department of Gynecology and Obstetrics), Christof Geisen, Markus Müller, Erhard Seifried, (Institute for Transfusion Medicine and Immunohematology)
Protestant Deaconess Hospital Freiburg: Christoph Wiesenack, Jürgen Ernst, Joachim Sauter (Department of Anaesthesiology)
Agatharied Hospital Hausham: Alexandra Bayer (Department of Anaesthesiology and Intensive Care Medicine)
SLK-Kliniken Heilbronn: Henry Weigt (Department of Anaesthesiology)
University Hospital Jena: Ansgar Raadts (Department of Anaesthesiology and Intensive Care Medicine)
University Hospital Schleswig-Holstein Kiel: Johannes Duemmler, Matthias Gruenewald, Lars Hummitzsch, Ulf Lorenzen, Jochen Renner, Maxim Sokirjanski, Markus Steinfath (Department of Anaesthesiology and Intensive Care), Matthias Pagel (Information-Technology UKSH), Thomas Puehler, Assad Haneya (Department of Cardiovascular Surgery), Ruwen Berndt, René Rusch (Department of Vascular Surgery), Julius Pochhammer, Thomas Becker (Department of General and Visceral Surgery), Tim Klueter, Andreas Seekamp (Department of Trauma, Hand and Reconstructive Surgery), Hajrullah Ahmeti, Ann-Kristin Helmers (Department of Neurosurgery), Daniar Osmonow (Department of Urology), Dirk Bauerschlag (Department of Gynecology and Obstetrics), Henning Wieker (Department of Oral Maxillofacial and Plastic Facial Surgery), Markus Hoffmann (Department of Otorhinolaryngology)
Klinikum Leverkusen: Jens Friedrich (Department of Anaesthesiology and Intensive Care Medicine)
Marienhaus Klinikum St.Wendel-Ottweiler: Martin Gutjahr, Martin Bier (Department of Anaesthesiology)
München Klinik Bogenhausen, Department of Anesthesiology, Operative Intensive Care Medicine and Pain Therapy, Munich: Patrick Friederich (Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy)
University Hospital Muenster: Andrea U. Steinbicker, Carola Wempe, Kira Kieserling, Marcel Rauer, Andreea Ceanga, Frank Peters, Diana Goncalves, Patrick Naber, Hugo K Van Aken, Alexander Zarbock (Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy), Walter Stummer (Department of Neurosurgery), Tobias Latal (Department of Medical Controlling), R. G. Geissler, H. Hillmann (Institute of Transfusion Medicine), Matthias Stelljes, Andrea Kerkhoff, Wolfgang Berdel, Georg Lenz (Medical Clinic A, Haematology, Pneumology, Haemostaseology and Oncology).
Diakonie Hospital Nuremberg: Klaus Schwendner (Department of Anaesthesiology and Operative Intensive Care Medicine)
Ortenau Klinikum Offenburg-Kehl: Josef Thoma (Department of Anaesthesiology and Intensive Care Medicine)
St. Josef-Stift Sendenhorst: Matthias Boschin (Department of Anaesthesiology and Intensive Care Medicine)
University Hospital Wuerzburg: Philipp Helmer, Sebastian Hottenrott, Adina Kleinerüschkamp, Peter Kranke, Patrick Meybohm, Daniel Roeder, Tobias Schlesinger, Benedikt Schmid, Magdalena Sitter, Jan Stumpner (Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine)
Klinikum Mittelmosel Zell: Patrick Stark* (Department of Visceral and Vascular Surgery) *former affiliation at time of data provision
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was conducted with internal department funding, and with support from B. Braun Melsungen, CSL Behring, Fresenius Kabi and Vifor Pharma for the implementation of Patient Blood Management program.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
ESC, PM, EG, FP, PB and KZ developed the medical study concept. ESC, FP and PM developed the statistical study design. ESC, MV, DN, CW, AB, HW, AR, MG, JF, MG, MGR, MW, PF, AUS, KS, JT and PS collected the data. ESC performed the statistical analysis and interpreted the results statistically. PM, PB, EG, KZ and FP interpreted the outcome medically. ESC, PM and FP prepared the first draft of the manuscript. All authors provided input and critical review of the manuscript leading to the final version. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University Hospital Frankfurt, Goethe University (first vote ref. 380/12 from 10 January 2013, amendments from 17 June 2013 to 1 June 2016, second vote ref. 318/17 from 30 November 2017), who waived the requirement for informed patient`s consent.
Consent for publication
Not applicable.
Competing interests
PM received honoraria for scientific lectures from Belgian Red Cross, Biotest, CSL Behring GmbH, Bundesamt für Bevölkerungsschutz und Katastrophenhilfe (BKK), Fresenius, Haemonetics, Landesärztekammer Sachsen, Landesärztekammer Hessen, Masimo, Radiometer, Schöchl medical Education, Thieme-Verlag, Trillium Diagnostik, Werfen GmbH, ViforPharma GmbH. PB received travel grants from Roche and Medac. KZ and his department received support from B. Braun Melsungen, CSL Behring, Fresenius Kabi, and Vifor Pharma for the implementation of Frankfurt‘s Patient Blood Management program and received honoraria for scientific lectures from CSL Behring, implatcast GmbH, med Update GmbH, Pharmacosmos and Vifor Pharma. MG received honoraria for scientific lectures from CSL Behring and Vifor Pharma. FP received honoraria from Pharmacosmos for scientific lectures.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patrick Stark is formerly affiliated to the Department of Visceral and Vascular Surgery, Klinikum Mittelmosel, Zell, Germany, at time of data provision.
This article is part of the Topical Collection on Neurosurgery general
Supplementary Information
ESM 1
(DOCX 42 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmitt, E., Meybohm, P., Neef, V. et al. Preoperative anaemia and red blood cell transfusion in patients with aneurysmal subarachnoid and intracerebral haemorrhage — a multicentre subanalysis of the German PBM Network Registry. Acta Neurochir 164, 985–999 (2022). https://doi.org/10.1007/s00701-022-05144-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05144-7