Abstract
Background
Deep surgical site infections (dSSIs) after instrumented spinal surgery pose major therapeutic challenges. Standard treatment involves surgical debridement, wound drainage, and long-term antibiotic administration. Autologous platelet-rich fibrin (PRF) constitutes a biomaterial obtained from patients’ own blood that contains leukocytes, chemokines and growth factors boosting cicatrization. Due to favorable results reported from other surgical disciplines such as dentistry, orthopedics, maxillofacial and plastic surgery using PRF, the authors hypothesized that PRF augmentation will promote wound healing in dSSIs.
Objective
To report our preliminary results on the safety and efficacy of autologous-PRF as an add-on therapy on a pilot case series of persistent dSSI after instrumented spinal surgery.
Methods
Among the 293 patients who underwent dorsal decompression and stabilization of the cervical, thoracic, and lumbar spine due to degenerative diseases in our department, 12 patients (4%) presented persisting dSSI after standard wound debridement and antibiotic treatment. PRF augmentation was used during a second surgical revision as an add-on therapy to standard debridement. In all cases, the wound was primarily closed without drains.
Results
Wound healing was completed between 14 and 21 days after the second surgical revision in all patients. At a median follow-up of 8 months (range: 6 to 18 months), no recurrence of dSSI nor complications were encountered in any case.
Conclusions
Our preliminary results suggest that PRF augmentation in persistent dSSI after instrumented spinal surgery appears to be a safe and effective strategy to promote wound healing. Prospective controlled studies are required to define the efficiency of PRF more clearly in both treating and preventing dSSI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical wound healing is a complex process influenced by both exogenous and endogenous factors such as: length of the incision, surgical duration, pathogens’ colonization with pathogens, diabetes, smoking, local biology/vascularization (e.g. revision surgery, skin affected by radiotherapy) and nutritional status [7]. With an incidence of up to 12%, post-operative spinal infections constitute a frequent complication [4, 6]. The Center for Disease Control and Prevention classifies the surgical site infections (SSIs) after spinal surgery into superficial and deep, located epifascially (skin and subcutaneous tissue), and subfascially (epidural abscess, spondylitis, spondylodiscitis), respectively [11, 32]. Deep SSIs is particularly observed after extensive exposures and metalwork implantation, often leading to soft tissue defects, impairing patients’ recovery with significant socio-economic costs [4, 6].
There are no well standardized management protocols for the treatment of dSSIs. Most widespread practices include extended wound debridement, closed-suction drains and identification of pathogens with subsequent targeted antibiotic treatment [6, 24, 31]. However, reconstruction of paravertebral soft tissue and wound healing may be challenging after extensive debridement, since necrotized space fills with a mixture of fluid and wound debris (Fig. 1,1B). This environment noticeably impairs cell-migration and proliferation leading to persistent dSSI, particularly in the presence of comorbidities interfering with the physiological wound healing [28]. Since primary wound closure frequently is not possible for these reasons, antimicrobial moisture-retentive dressings and vacuum therapies are commonly applied [28].
A Axial section of a healthy lumbar spine, i, Vertebral body; ii, paravertebral structures; iii, Fascia; iv, subcutaneous fat; v, dermis and subcutis. B-I Deep surgical site infection with tissue destruction. Left and right to the spinal process are represented the implanted posterior instrumentation. C Axial view after extensive debridement and resection of infected and necrotic tissues. The residual tissue defect is C-II. The autologous platelet-rich fibrin matrix is green. D Platelet rich fibrin augmented tissue defect after re-adaptation of the paravertebral structures and primary wound closure
Platelet-rich fibrin (PRF) constitutes an autologous biomaterial obtained from the patients’ own peripheral blood after centrifugation. Consisting of a fibrin matrix containing high concentrations of platelet-derived growth factors, chemokines, and leukocytes were able to enhance wound healing and to provide local antimicrobial protection [13, 16, 17, 29]. Due to these biological properties, PRF was used in restorative dentistry, orthopedics, maxillofacial and plastic surgery, as well as for the treatment of persistent diabetic ulcerations with promising results [2, 5, 33, 14, 22, 30]. Therefore, we hypothesized that PRF may be an appropriate biomaterial to promote wound healing in deep spinal infections which failed to respond to the traditional surgical approach (wound debridement, drains, antibiotics), especially in the presence of spinal metalwork. Favorable results observed in our consecutive case series, encouraged us to report on our findings.
Patients and methods
Patients
During a two-year period in our department (12/2017–11/2019), a total of 293 patients underwent dorsal decompression and stabilization of the cervical, thoracic, and lumbar spine due to degenerative pathology. Surgical site infections (SSIs) occurred in 24 patients (8%), of which 4 (1%) were superficial and 20 (7%) were deep infections. While superficial infections resolved after conservative treatment with antibiotics in every case, all patients with deep surgical site infections (dSSIs) underwent extended surgical debridement (first surgical revision) and intravenous (IV) pathogen-specific antibiotic therapy. Although in 8/20 cases, dSSI resolved with this therapeutic strategy, 12/20 patients (4%) failed to respond with persistent purulent secretion and wound dehiscence, thus were included in our study. After another debridement (second revision) and continuous antibiotic therapy, PRF augmentation was performed as an add-on therapy in these 12 patients. Details on surgical procedures and PRF augmentation are given in the respective paragraphs.
Initial surgery
Of the 12 patients, 6 (50%) were operated in the cervical, 5 (42%) in the lumbar and 1 (8%) in the thoracic spine via a dorsal approach, which was minimally-invasive in 4 cases (33%). Titanium implants were used across the cohort. According to our local policy, patients received as prophylaxis during the anesthetic induction 2 g Cefuroxime or alternatively 500 mg Clindamycin, if allergic to penicillin and if needed repeated intraoperatively every 4 h and 8 h, respectively. The surgical site was cleaned with Betadine or Chlorhexidine scrub. During revision, dural tear was identified in 5 patients (41.7%). Surgical drains were placed and removed when the drainage was less than 50 ml/day.
First surgical revision
Deep SSI was confirmed by contrast-enhanced MRI and laboratory tests including blood cell counts, erythrocyte sedimentation rate and C-reactive protein. The germ spectrum was determined by wound swabs and blood cultures. Extensive wound washout with Ringer´s solution and meticulous surgical debridement of all devitalized tissue was performed in every case. Wound was primarily closed in layers, leaving in situ two drains. In all cases the metalwork was preserved and a pathogen-specific antibiotic therapy was performed.
During the 4 weeks follow-up period, this standard approach failed to resolve the dSSI in 12 cases as confirmed by clinical laboratory and radiological assessments. Therefore, PRF augmentation was planned as an add-on therapy during the second debridement after acquiring patients’ informed consent.
Preparation of platelet-rich fibrin (PRF) as solid (s-PRF) and injectable (i-PRF) biomaterial
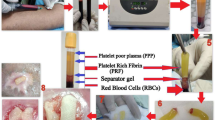
For the preparation of PRF, 120 ml of patient’s own peripheral blood was collected intraoperatively using a standard butterfly. The blood was centrifuged in a DUO centrifuge at 1200 rpm for 8 min (177 g) using a fixed angled rotor with a 110 mm radius (Process for PRF, Nice, France) according to the low centrifugation concept protocol of Choukroun and Ghanaati [13]. This protocol ensures a high concentration and homogenous distribution of autologous platelet-derived growth factors in PRF [13]. For every wound augmentation, 6 s-PRF vials and 6 i-PRF vials were used, each containing 10 ml of donor-matched blood in glass and plastic tubes, respectively (Process for PRF, Nice, France). After the completion of the centrifugation cycle, the tubes were left in the device to settle for 5 min.
Second surgical revision
After renewed debridement, the residual tissue defect was augmented with autologous PRF in both solid and injectable forms (Fig. 1, 1C, 1D). From the glass tubes, s-PRF was collected (Fig. 2, 2A, 2B) and inserted in the tissue-defect (Fig. 3, 3B). From the plastic tubes, the thick fluid injectable PRF (i-PRF) was aspired in a 20-ml syringe and filled the remaining tissue-defect (Fig. 2, 2C, 2D).
Representative case from our study group. Craniocervical posterior fixation was performed. A Intraoperative posterior view of the deep surgical site infection. The instrumentation is visible. B After extensive debridement, the residual tissue defect was augmented with solid and injectable platelet-rich fibrin. Primary wound closure was performed without closed suction drains. C and D Lateral and anteroposterior view of the cervical spine 19 days after platelet-rich fibrin augmentation
Revision of spinal instrumentation was not required in any case and drains or local antibiotics were not used. Primary wound closure was performed in every patient. The pathogen-specific IV antibiotic regimen was continued. After the normalization of the C-reactive protein (CRP) values, the IV antibiotics were switched to oral continuing for 2 more weeks.
Subsequent follow-ups for wound checks and laboratory tests were performed twice a month for 3 months, including clinical, neurological assessments and CT/MR imaging, as needed. All patients were scheduled to have CT-scans 12 months postoperatively to evaluate bone fusion. Observation time ranged from 6 to 18 months (median: 8 months).
Results
There were 7 females and 5 males with a mean age of 66.6 ± 5 year. Obesity defined as a body mass index (BMI) > 30 was observed in 7 (58.3%) cases. Four patients (33.3%) had diabetes.
Staphylococcus aureus was identified as the leading pathogen by swabs and blood cultures in all 12 patients at the first surgical revision and all of them received tailored antibiotic therapy.
The postoperative course after the second surgical revision with PRF augmentation was uneventful in every patient and completed wound healing was achieved between 14 and 21 days in all cases. Postoperative in-hospital stay was 18.3 ± 2.7 days. No recurrent SSI and no complications were noted at a median follow-up of 8 months (range: 6–18 months) in any case. Postoperative CT-scans at 12 months revealed distinct signs of bony fusion in all cases. Loosening of single screw was noted in 2 patients, however no surgical intervention was required, since they were virtually asymptomatic and presenting sings of advanced bony fusion on CT scans. At last follow-up, all patients presented satisfying outcome without additional neurological deficits and normal inflammation parameters.
Discussion
Instrumented spinal surgery is a commonly utilized procedure, which however is not devoid of complications, especially when it comes to patients with associated comorbidities or elderly, which could lead to surgical site infections (SSIs) [8]. Foremost, diabetes-associated systemic microvascular impairment can reduce the local ability to resist infection [19], and leukocyte functions (adherence, chemotaxis and phagocytosis) are undermined by hyperglycemia [9]. Furthermore, diabetes can disturb the collagen synthesis and fibroblast proliferation, thus delaying the wound healing process [3]. Nicotine induces peripheral vasoconstriction and tissue hypoxia resulting in impaired local angiogenesis and epithelialization [23]. Obesity, surgical invasiveness, and implantation of foreign material have been identified as independent risk factors for deep SSIs (dSSIs) [15]. Thus, dSSIs constitute a major problem in instrumented spinal surgery, especially in elderly patients when larger exposures are required.
Favorable results in other disciplines such as dentistry, orthopedics, plastic and maxillofacial surgery, as well as in the treatment of persistent diabetic ulcerations [2, 5, 33, 14, 22, 30] encouraged us to focus on the use of biomaterials with high concentrations of autologous platelet-derived growth factors that can augment the residual tissue defect and provide an active cell-migration matrix. Platelet-derived growth factors such as vascular endothelial growth factor (VEGF), keratinocyte growth factor (KGF), insulin-like growth factor (IGF), epidermal growth factor (EGF) and transforming growth factor-β (TGF-β) are found at high concentrations and homogeneously distributed in the fibrin matrix of platelet-rich fibrin (PRF), from which they are slowly released into the surrounding tissue [16, 29, 14, 18]. Schar et al. [29] showed that PRF-associated growth factors, known as key coordinators of the physiological wound healing cascade, can enhance the wound cell-migration promoting healing. Raposio et al. [27] demonstrated already the efficacy of PRF augmentation on wound healing in chronic venous, diabetic and ischemic skin ulcerations compared to standard approaches. In a prospective study including 44 patients with persistent venous leg and diabetic foot ulcerations, Pinto et al. [26] emphasized the regenerative properties of PRF applied as monotherapy and the important role of patients’ own leucocytes. In addition, Vaheb et al. [34] demonstrated in a randomized, placebo-controlled, triple-blind study that PRF augmentation of split-thickness skin grafting in burns and chronic wounds significantly improved healing rates.
This pilot study reports for the first time the use of PRF for the treatment for dSSI after spinal instrumented surgery. Complete wound healing was achieved in all of our 12 consecutive patients between 14 and 21 days after the second revision using PRF. At a median follow-up of 8 months (range, 6–18 months), no recurrence of dSSI was observed and no complications were encountered in any case. In addition, instrumentation was preserved in all patients.
Readers might argue that the second surgical debridement alone combined with continuous pathogen-specific antibiotic treatment could explain the elimination of the dSSI and successful wound healing in our cases. However, large studies evaluating management strategies for dSSIs after spinal surgery with preservation of the instrumentation consistently reported the necessity of multiple surgical revisions, the use of antimicrobial moisture-retentive dressings and vacuum therapy as well as prolonged antibiotic treatment over months to achieve wound healing [1, 10, 12, 20, 21, 25]. Conversely, complete wound healing has observed within max 21 days in this cohort after the second surgical revision indicating a specific effect from PRF.
Limitations of this pilot study could be the small cohort and its retrospective design, which however could be understandable considering that in a 2-year period only 12 infected patients out of 24 needed an adjuvant PRF approach to achieve healing. The present study has not utilized an external matched control, which appears to be a common denominator with most of the published studies. The authors recognize the need for randomized controlled trials to demonstrate the efficacy of PRF to both treat and prevent dSSI after instrumented spinal surgery.
Conclusions
This pilot single-center retrospective consecutive case series of patients with persistent deep SSI after instrumented spinal surgery suggests that PRF augmentation as an add-on therapy to standard debridement is safe and might provide a promising tool to achieve wound healing in critical cases. Using autologous biomaterial, PRF augmentation constitutes a simple and low-cost technique that can be easily applied in every operating room.
References
Alcala-Marquez C, Torrealba R, Mehbod AA, Dawson JM, Dressel TD, Transfeldt EE (2018) Recurrent surgical site infections in the spine after treatment with the vacuum-assisted closure (VAC) system. Clin Spine Surg 31(8):351–355
Alzahrani AA, Murriky A, Shafik S (2017) Influence of platelet rich fibrin on post-extraction socket healing: A clinical and radiographic study. Saudi Dent J 29(4):1–7
Argyropoulos AJ, Robichaud P, Balimunkwe RM, Fisher GJ, Hammerberg C, Yan Y, Quan T (2016) Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged-appearing skin. PLoS ONE 11(4):e0153806
Beiner JM, Grauer J, Kwon BK, Vaccaro AR (2003) Postoperative wound infections of the spine. Neurosurg Focus 15(3):E14
Bielecki T, Dohan Ehrenfest DM (2012) Platelet-rich plasma (PRP) and Platelet-Rich Fibrin (PRF): surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr Pharm Biotechnol 13(7):1121–1130
Blumberg TJ, Woelber E, Bellabarba C, Bransford R, Spina N (2018) Predictors of increased cost and length of stay in the treatment of postoperative spine surgical site infection. Spine J 18(2):300–306
Bowler PG (2002) Wound pathophysiology, infection and therapeutic options. Ann Med 34(6):419–427
Bowler PG (2009) Wound pathophysiology, infection and therapeutic options. Ann Med 34(6):419–427
Butler SO, Btaiche IF, Alaniz C (2005) Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy 25(7):963–976
Chaichana KL, Bydon M, Santiago-Dieppa DR, Hwang L, McLoughlin G, Sciubba DM, Wolinsky J-P, Bydon A, Gokaslan ZL, Witham T (2014) Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. J Neurosurg Spine 20(1):45–52
Chaudhary SB, Vives MJ, Basra SK, Reiter MF (2007) Postoperative spinal wound infections and postprocedural diskitis. J Spinal Cord Med 30(5):441–451
Chen K, Lin J-T, Sun S-B, Lin J, Kong J-Z, Tian N-F (2018) Vacuum-assisted closure combined with a closed suction irrigation system for treating postoperative wound infections following posterior spinal internal fixation. J Orthop Surg Res 13(1):321–328
Choukroun J, Ghanaati S (2018) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg 44(1):87–95
Cieslik-Bielecka A, Choukroun J, Odin G, Dohan Ehrenfest DM (2012) L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds. Curr Pharm Biotechnol 13(7):1266–1277
Cizik AM, Lee MJ, Martin BI, Bransford RJ, Bellabarba C, Chapman JR, Mirza SK (2012) Using the spine surgical invasiveness index to identify risk of surgical site infection: a multivariate analysis. J Bone Joint Surg Am 94(4):335–342
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e45-50
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e51
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J (2017) Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J Periodontol 88(1):112–121
He Z, King GL (2004) Microvascular complications of diabetes. Endocrinol Metab Clin North Am 33(1):215-38 xi-xii
Kale M, Padalkar P, Mehta V (2017) Vacuum-assisted closure in patients with post-operative infections after instrumented spine surgery: a series of 12 cases. J Orthop Case Rep 7(1):95–100
Maruo K, Berven SH (2014) Outcome and treatment of postoperative spine surgical site infections: predictors of treatment success and failure. J Orthop Sci 19(3):398–404
Miron RJ, Zucchelli G, Pikos MA et al (2017) Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Invest 21(6):1913–1927
Misery L (2004) Nicotine effects on skin: are they positive or negative? Exp Dermatol 13(11):665–670
Mitra A, Mitra A, Mitra A, Harlin S (2004) Treatment of massive thoracolumbar wounds and vertebral osteomyelitis following scoliosis surgery. Plast Reconstr Surg 113(1):206–213
Patel H, Khoury H, Girgenti D, Welner S, Yu H (2017) Burden of surgical Site infections associated with select spine operations and involvement of staphylococcus aureus. Surg Infect (Larchmt) 18(4):461–473
Pinto NR, Ubilla M, Zamora Y, Del Rio V, Dohan Ehrenfest DM, Quirynen M (2018) Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: a prospective cohort study. Platelets 29(5):468–475
Raposio E, Bertozzi N, Bonomini S, Bernuzzi G, Formentini A, Grignaffini E, Pio Grieco M (2016) Adipose-derived stem cells added to platelet-rich plasma for chronic skin ulcer therapy. Wounds 28(4):126–131
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49(1):35–43
Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D (2015) Platelet-rich Concentrates Differentially Release Growth Factors and Induce Cell Migration In Vitro. Clin Orthop Relat Res 473(5):1635–1643
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ (2018) Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 00(00):1–8
Watanabe M, Sakai D, Matsuyama D, Yamamoto Y, Sato M, Mochida J (2010) Risk factors for surgical site infection following spine surgery: efficacy of intraoperative saline irrigation. J Neurosurg Spine 12(5):540–546
Weinstein MA, McCabe JP, Cammisa FP (2000) Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 13(5):422–426
Carreon LY, Glassman SD, Anekstein Y, Puno RM (2005) Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine 30(9):E243–6– discussion E247
Vaheb M, Karrabi M, Khajeh M, Asadi A, Shahrestanaki E, Sahebkar M (2020) Evaluation of the effect of platelet-rich fibrin on wound healing at split-thickness skin graft donor sites: a randomized, placebo-controlled, triple-blind study. Int J Low Extrem Wounds 1534734619900432
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the local ethics committee (176/20). All procedures and applications on patients were in accordance with the institutional ethical standards as well as the 1964 Helsinki declaration and its later amendments. Informed consent was acquired from all patients.
Conflict of interest
Ioannis Vasilikos, Roland Roelz, Christoph Scholz, Boris Mizaikoff, Katerina Argiti, Watzlawick Ralf, Georgios-Christos Giagkos, Evangelos M. Fragkakis, Jürgen Beck, Ulrich Hubbe.
The above listed authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Prof. Ghanaati is a medical consultant in Mectron Inc. that produces the PRF-Centrifugation Device used in our experiments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Spine-Other
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasilikos, I., Roelz, R., Scholz, C. et al. Autologous platelet-rich fibrin (PRF) augmentation as an add-on therapy in deep surgical site infections (dSSIs) after instrumented spinal surgery: preliminary results of a single institution case series. Acta Neurochir 163, 2761–2767 (2021). https://doi.org/10.1007/s00701-021-04952-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04952-7