Abstract
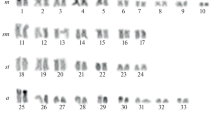
Fourteen North American members of the “Xanthocephalum group” were studied by classical and molecular cytogenetics. Location and number of rDNA sites were determined by FISH. For the 5S rDNA, a probe was obtained from Prionopsis ciliata. Most species were diploid (2n = 12), although Isocoma menziesii, Grindelia hirsutula, G. robusta, both varieties of G. stricta, and one population of G. camporum were tetraploid (2n = 24). Diploid Grindelia and Prionopsis ciliata were 5m + 1sm, tetraploids 10m + 2sm, except G. hirsutula (8m + 4sm), and Isocoma and Olivaea 6m + 2sm and 3m + 3sm, respectively. Most species had satellites on the short arms of m pairs: two in tetraploids and P. ciliata and one in diploids. Satellites were associated with two CMA+/DAPI− bands in diploid species and four bands in tetraploids and in P. ciliata. rDNA loci (two in diploids to four in tetraploids) may be indicative of ploidy level. Grindelia tetraploids could have originated recently by autopolyploidy. Chromosome duplication was followed by modifications in the genome structure, resulting in higher heterochromatin amounts not associated with NORs. There is only one 5S site per basic genome in para or pericentromeric regions. Although not always large, chromosome variation has accompanied the evolutionary divergence of the taxa studied.




Similar content being viewed by others
References
Adams KL, Wendel JF (2005) Polyploidy and genome evolution in plants. Curr Opin Plant Biol 8:135–141
Adams SP, Leitch IJ, Bennet MD, Chase MW, Leitch AR (2000) Ribosomal DNA evolution and phylogeny in Aloe (Asphodelaceae). Am J Bot 87:1578–1583
Baeza C, Schrader O (2005) Comparative karyotype analysis in Haplopappus Cass. and Grindelia Willd. (Asteraceae) by double FISH with rRNA specific genes. Plant Syst Evol 251:161–172
Bartoli A, Tortosa R (1998) Estudios cromosómicos en especies sudamericanas de Grindelia (Astereae, Asteraceae). Kurtziana 26:165–171
Battaglia E (1955) Chromosome morphology and terminology. Caryologia 8:179–187
Besendorfer V, Samardzija M, Zoldos V, Solic ME, Papes D (2002) Chromosomal organization of ribosomal genes and NOR-associated heterochromatin, and NOR activity in some populations of Allium commutatum Guss. (Alliaceae). Bot J Linn Soc 139:99–108
Cai Q, Zhang D, Liu Z-L, Wang X-R (2006) Chromosomal localization of 5S and 18S rDNA in five species of subgenus Strobus and their implications for genome evolution of Pinus. Ann Bot 97:715–722
Carr GD, King RM, Powell AM, Robinson HE (1999) Chromosome numbers in Compositae. XVIII. Am J Bot 86:1003–1013
Cerbah M, Coulaud J, Godelle B, Siljak-Yakovlev S (1995) Genome size, fluorochrome banding, and karyotype evolution in some Hypochaeris species. Genome 38:689–695
Cheng Z, Presting GG, Buell CR, Wing RA, Jiang J (2001) High-resolution pachytene chromosome mapping of bacterial artificial chromosomes anchored by genetic markers reveals the centromere location and the distribution of genetic recombination along chromosome 10 of rice. Genetics 157:1749–1757
Clarkson JJ, Lim KY, Kovarik A, Chase MW, Knapp S, Leitch AR (2005) Long-term genome diploidization in allopolyploid Nicotiana section Repandae (Solanaceae). New Phytol 168:241–252
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Coşkuncelebi K, Hayirlioğlu-Ayaz S (2006) Notes on chromosome numbers and karyotypes of five species in Hieracium L. s.str. (Asteraceae) from Turkey. Caryologia 59:19–24
D’Amato G (2000) Speckled Fluorescent Banding Pattern in Scorzonera (Asteraceae). Hereditas 132:265–267
De Jong DC, Beaman JH (1963) The genus Olivaea (Compositae, Astereae). Brittonia 15:86–92
Deumling B, Greilhuber J (1982) Characterization of heterochromatin in different species of the Scilla siberica group (Liliaceae) by in situ hybridization of satellite DNAs and fluorochrome banding. Chromosoma 84:535–555
Dimitrova D, Greilhuber J (2000) Karyotype and DNA content evolution in ten species of Crepis (Asteraceae) distributed in Bulgaria. Bot J Linn Soc 132:281–297
Dunford MP (1964) A cytogenetic analysis of certain polyploids in Grindelia (Compositae). Am J Bot 51:49–56
Dunford MP (1969) Chromosome numbers of six Texas species of Grindelia and meiotic analysis of some interspecific hybrids. J Colorado Wyoming Acad Sci 6:20
Dunford MP (1986) Chromosome relationships of diploid species of Grindelia (Compositae) from Colorado, New Mexico and adjacent areas. Am J Bot 73:297–303
Fregonezi JN, Fernandes T, Domingues Torezan JM, Vieira O, Vanzela ALL (2006) Karyotype differentiation of four Cestrum species ( Solanaceae ) based on the physical mapping of repetitive DNA. Genet Mol Biol 29:97–104
Fregonezi JN, Rocha C, Torezan JMD, Vanzela ALL (2004) The occurrence of different Bs in Cestrum intermedium and C. strigilatum (Solanaceae) evidenced by chromosome banding. Cytogenet Genome Res 106:184–188
Galasso I, Sublimi Saponetti L, Pignone D (1997) Cytotaxonomic studies in Vigna: 3. Chromosomal distribution and reacting properties of the heterochromatin in five wild species of the section Vigna. Caryologia 49:311–319
Garcia S, Lim KY, Chester M, Garnatje T, Pellicer J, Vallés J, Leitch AR, Kovařík A (2009) Linkage of 35S and 5S rRNA genes in Artemisia (family Asteraceae): first evidence from angiosperms. Chromosoma 118:85–97
Gerlach WL, Bedbrook JL (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Goodspeed TH (1954) The genus Nicotiana. Waltham MA, USA
Gottlob-McHugh SG, Ldvesque M, MacKenzie K, Olson M, Yarosh O, Johnson DA (1990) Organization of the 5S rRNA genes in the soybean Glycine max (L). Merrill and conservation of the 5S rDNA repeat structure in higher plants. Genome 33:486–494
Grau J (1976) Chromosomenzahlen von Südamerikanischen Haplopappus Arten. Mitt Bot Staatssamml München 12:403–410
Greilhuber J, Ehrendorfer F (1988) Karyological approaches to plant taxonomy. Animal Plant Sci 1:289–297
Guerra M (2000) Patterns of heterochromatin distribution in plant chromosomes. Genet Mol Biol 23:1029–1041
Hasterok R, Wolny E, Hosiawa M, Kowalczyk M, Kulak-Ksiazczyk S, Ksiazczyk T (2006) Comparative analysis of rDNA distribution in chromosomes of various species of Brassicaceae. Ann Bot 97:205–216
Hemleben V, Werts D (1988) Sequence organization and putative regulatory elements in the 5S rRNA genes of two higher plants (Vigna radiata and Matthiola incana). Gene 62:165–169
Huziwara Y (1967) Chromosomal evolution in Aster and related genera. Taxon 16:303–304
Jackson RC, Dimas CT (1981) Experimental evidence for systematic placement of the Haplopappus phyllocephalus complex (Compositae). Syst Bot 6:8–14
Jiang J, Gill BS (1994) Nonisotopic in situ hybridization and plant genome mapping: the first 10 years. Genome 37:717–725
Jiang J, Gill BS (2006) Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 49:1057–1068
Jones AG (1985) Chromosomal feature as generic criteria in the Astereae. Taxon 34:44–54
Jones RN, Houben A (2003) B chromosomes in plants: escapees from the A chromosome genome? Trends Plant Sci 8:417–423
Jones RN, Viegas W, Houben A (2008) A century of B chromosomes in plants: So What? Ann Bot 101:767–775
Jong J (1997) Laboratory manual of plant cytological techniques. Royal Botanical Garden, Edinburgh
Kamari G (1992) Karyosystematic studies on three Crepis species (Asteraceae) endemic to Greece. Plant Syst Evol 182:1–19
Kato A, Lamb JC, Birchler JA (2004) Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc Natl Acad Sci USA 101:13554–13559
Kulak S, Hasterok R, Maluszynska J (2002) Karyotyping of Brassica amphidiploids using 5S and 25S rDNA as chromosome markers. Hereditas 137:79–80
Lane MA (1993) Grindelia (gumplant). In: Hickman JC (ed) The Jepson manual: higher plants of California. University California Press, California
Lane MA, Li JW (1993) Documented chromosome numbers 1993:1. Chromosome number reports in Compositae with emphasis on Tribe Astereae of the Southwestern United States and Mexico. Sida 15:539–546
Lane MA, Hartman RL (1996) Reclassification of North American Haplopappus (Compositae, Astereae) completed: Rayjacksonia gen. nov. Am J Bot 83:356–370
Lane MA, Morgan DR, Suh Y, Simpson BB, Jansen RK (1996) Relationships of North American genera of Astereae, based on chloroplast DNA restriction site data. In: Hind DJN, Beentje HJ (eds) Compositae, systematics—proceedings of International Compositae Conference, Kew 1994, vol 1. Royal Botanic Gardens, UK, pp 49–77
Levan A, Fredga K, Sandberg A (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Lim KY, Matyasek R, Kovarík A, Leitch A (2004) Genome evolution in allotetraploid Nicotiana. Biol J Linn Soc 82:599–606
Liu B, Brubaker CL, Mergaei G, Cronn RC, Wendel JF (2001) Polyploid formation in cotton is not accompanied by rapid genomic changes. Genome 43:874–880
Mandákova T, Münzbergová Z (2006) Distribution and ecology of cytotypes of the Aster amellus aggregates in the Czech republic. Ann Bot 98:845–856
Marcon BA, Guerra M (2005) Variation in chromosome numbers, CMA bands and 45S rDNA sites in species of Selaginella (Pteridophyta). Ann Bot 95:271–276
McLaughlin S (1986) Defferentiation among populations of tetraploid Grindelia camporum. Am J Bot 17:1748–1754
Miranda M, Ikeda F, Endo T, Morigucki T, Omura M (1997) Comparative analysis on the distribution of heterochromatin in Citrus, Poncirus and Fortunella chromosomes. Chromosome Res 5:86–92
Moore A, Bartoli A, Tortosa R, Baldwin B (2012) Phylogeny, biogeography, and chromosome evolution of the amphitropical genus Grindelia (Asteraceae) inferred from nuclear ribosomal and chloroplast sequence data. Taxon 61:211–230
Morgan DR, Simpson BB (1992) A systematic study of Machaeranthera (Asteraceae) and related groups using restriction site analysis of chloroplast DNA. Syst Bot 17:511–531
Morgan DR (1997) Reticulate evolution in Machaeranthera (Asteraceae). Syst Bot 22:599–615
Morgan (2003) nrDNA external transcribed spacer (ETS) sequence data, reticulate evolution, and the systematics of Machaeranthera (Asteraceae). Syst Bot 28:179–190
Morton JK (1981) Chromosome numbers in Compositae from Canada and the U.S.A. J Linn Soc Bot 82:357–368
Moscone EA, Klein F, Lambru M, Fuchs J, Schweizer D (1999) Quantitative karyotyping and dual-colour FISH mapping of 5S and 18S-25S rDNA probes in the cultivated Phaseolus species (Leguminosae). Genome 42:1224–1233
Moscone EA, Lambrou M, Ehrendorfer F (1996) Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). Pl Syst Evo 202:37–63
Nesom GL (1978) Chromosome number in Erigeron and Conyza (Compositae). Sida 7:375–381
Nesom GL (1991) Taxonomy of Isocoma (Compositae, Astereae). Phytologia 70:69–114
Nesom GL (1993) Prionopsis (Asteraceae: Astereae) united with Grindelia. Phytologia 75:341–346
Nesom GL (1994) Subtribal classification of the Astereae (Asteraceae). Phytologia 76:193–274
Nesom GL (1997) Synopsis of Stephanodoria (Asteraceae:Astereae). Phytologia 82:107–113
Nesom GL (2000) New subtribes for North American Astereae (Asteraceae). Sida 19:263–268
Nesom GL, Suh Y, Simpson BB (1993) Prionopsis (Asteraceae: Astereae) united with Grindelia. Phytologia 75:341–346
Parisod C, Alix K, Just J, Petit M, Sarilar V, Mhiri C, Ainouche M, Chalhoub B, Grandbastien MA (2010) Impact of transposable elements on the organization and function of allopolyploid genomes. New Phytol 186:37–45
Pires JC, Zhao J, Schranz ME, Leon EJ, Quijada PA, Lukens LN, Osborn TC (2004) Flowering time divergence and genomic rearrangements in resynthesized polyploids (Brassica). Biol J Linn Soc 82:675–688
Raskina O, Barber JC, Nevo E, Belyayev A (2008) Repetitive DNA and chromosomal rearrangements: speciation-related events in plant genomes. Cytogenet Genome Res 120:351–357
Raven PH, Solbrig OT, Kyhos DW, Snow R (1960) Chromosome numbers in Compositae. I Astereae. Am J Bot 47:124–132
Riesenberg LH (2001) Chromosomal rearrangements and speciation. Trends Eco Evo 16:351–358
Romero Zarco C (1986) A new method for estimating karyotype asymmetry. Taxon 35:526–530
Röser M (1994) Pathways of karyological differentiation in palms (Arecaceae). Plant Syst Evol 189:83–122
Ruas CF, Vanzela ALL, Santos MO, Fregonezi JN, Ruas PM, Matzenbacher NI, AguiarPerecin MLR (2005) Chromosomal organization and phylogenetic relationship in Hypochaeris species (Asteraceae) from Brazil. Genet Molec Biol 28:129–139
Ruas PM, Ruas CF, Maffei MD, Marin-Morales MA, Aguiar-Perecin MLR (2000) Chromosome studies in the genus Mikania (Asteraceae). Genet Molec Biol 23:979–984
Sastri DCK, Hilt R, Appels ES, Lagudah J, Playford BR, Baum BR (1992) An overview of evolution in plant 5S DNA. Pl Syst Evo 183:169–181
Schwarzacher T (2003) DNA, chromosomes, and in situ hybridization. Genome 46:953–962
Schwarzacher T, Heslop-Harrison P (2000) Practical in situ hybridization. Bios Scientific Publishers Limited, Oxford
Schwarzacher T, Ambros P, Schweizer D (1980) Application of Giemsa banding to orchid karyotype analysis. Plant Syst Evol 134:293–297
Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58:307–324
Schweizer D, Ambros P (1994) Chromosome banding. In: Gosden JR (ed) Methods in molecular biology. Chromosome analysis protocols. Humana Press, Totowa
Semple J (2008) Cytotaxonomy and cytogeography of the goldenaster genus Heterotheca (Asteraceae: Astereae). Botany 86:886–900
Semple JC (1980) IOPB Chromosome number reports LXVII. Taxon 29:357–358
Semple JC, Chmielewski JG, Lane M (1989) Chromosome number determinations in fam. Compositae, tribe Astereae. III. Additional counts and comments on generic limits and ancestral base numbers. Rhodora 91:296–314
Semple JC, Chmielewski JG, Xiang C (1992) Chromosome number determinations in fam. Compositae, tribe Astereae. IV. Additional reports and comments on the cytogeography and status of some species of Aster and Solidago. Rhodora 94:48–62
Solbrig O (1964) Chromosome number in compositae V. Astereae II. Am J Bot 51:513–519
Sumner AT (1990) Chromosome banding. Unwin Hyman Limited, London
Taketa S, Harrison GE, Heslop-Harrison JS (1999) Comparative physical mapping of the 5S and 18S-25S rDNA in nine wild Hordeum species and cytotypes. Theor Appl Genet 98:1–9
Torrell M, Cerbah M, Siljak-Yakovlev S, Valles J (2003) Molecular cytogenetics of the genus Artemisia (Asteraceae, Anthemideae): fluorochrome banding and fluorescence in situ hybridization. I. Subgenus Seriphidium and related taxa. Plant Syst Evol 239:141–153
Urdampilleta J, Amat A, Bidau C (2005) Karyotypic studies and morphological analysis of some reproductive features in five species of Conyza (Astereae: Astereceae) from northeastern Argentina. Bol Soc Arg Bot 40:91–99
Vallès J, McArthur ED (2001) Artemisia systematics and phylogeny: Cytogenetic and molecular insights. In: McArthur ED, Fairbanks DJ (eds) (comp) Shrubland ecosystem genetics and biodiversity. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Provo, Utah
Vanzela ALL, Ruas CF, Oliveira MF, Ruas PM (2003) Characterization of diploid, tetraploid and hexaploid Helianthus species by chromosome banding and FISH with 45S rDNA probe. Genetica 114:105–111
Watanabe K, Yahara T, Denda T, Kosuge K (1999) Chromosomal evolution in the genus Brachyscome (Asteraceae, Astereae): statistical tests regarding correlation between changes in karyotype and habit using phylogenetic information. J Plant Res 112:145–161
Whitaker T, Steyermark J (1935) Cytological aspects of Grindelia species. Bull Torrey Bot Club 62:69–73
Acknowledgments
Dr Carolyn Ferguson (KSU Herbarium), Biol. Abigail Moore (JEPS Herbarium), and Dr José Luis Villaseñor (Instituto de Biología, UNAM) kindly send the samples. Grants from “Consejo Nacional de Investigaciones Científicas y Técnicas” (CONICET, Argentina), FONCYT, and “Universidad Nacional de Córdoba” (SECyT, Argentina) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno, N.C., Stiefkens, L., Las Peñas, M.L. et al. Molecular cytogenetic studies of the “Xanthocephalum group” (Asteraceae). Plant Syst Evol 298, 1503–1514 (2012). https://doi.org/10.1007/s00606-012-0653-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-012-0653-1