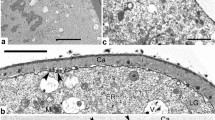
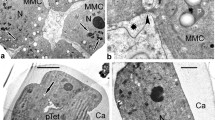
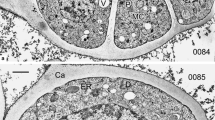
Abstract
Compositae exhibit some of the most complex and diverse pollen grains in flowering plants. This paper reviews the evolutionary and developmental origins of this diversity in pollen structure using recent models based on the behaviour of colloids and formation of micelles in the differentiating microspore glycocalyx and primexine. The developmental model is consistent with observations of structures recovered by pollen wall dissolution. Pollen wall diversity in Compositae is inferred to result from small changes in the glycocalyx, for example ionic concentration, which trigger the self-assembly of highly diverse structures. Whilst the fine details of exine substructure are, therefore, not under direct genetic control, it is likely that genes establish differences in the glycocalyx which define the conditions for self-assembly. Because the processes described here for Compositae can account for some of the most complex exine structures known, it is likely that they also operate in pollen walls with much simpler organisation.








Similar content being viewed by others
References
Barnes SH, Blackmore S (1986) Some functional features during pollen development. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 71–80
Barnes SH, Blackmore S (1988) Pollen ontogeny in Catananche caerulea L. (Compositae: Lactuceae) II. Free microspore stage to the formation of the male germ unit. Ann Bot 62:615–623
Blackmore S (1981) Palynology and intergeneric relationships in subtribe Hyoseridinae (Compositae: Lactuceae). Bot J Linn Soc 82:1–13
Blackmore S (1982) Palynology of subtribe Scorzonerinae (Compositae: Lactuceae) and its taxonomic significance. Grana 21:149–160
Blackmore S (1990) Sporoderm homologies and morphogenesis in land plants, with a discussion of Echinops sphaerocephala (Compositae). Pl Syst Evol (suppl 5):1–12
Blackmore S, Barnes SH (1985) Cosmos pollen ontogeny: a scanning electron microscope study. Protoplasma 126:91–99
Blackmore S, Barnes SH (1987) Pollen wall morphogenesis in Tragopogon porrifolius (Compositae: Lactuceae) and its taxonomic significance. Rev Palaeobot Palynol 52:233–246
Blackmore S, Barnes SH (1988) Pollen ontogeny in Catananche caerulea L. (Compositae: Lactuceae) I. Premeiotic phase to establishment of tetrads. Ann Bot 62:605–614
Blackmore S, Claugher D (1987) Observations on the substructural organisation of the exine in Fagus sylvatica L. (Fagaceae) and Scorzonera hispanica L. (Compositae: Lactuceae). Rev Palaeobot Palynol 53:175–184
Blackmore S, Crane PR (1988) Systematic implications of pollen and spore ontogeny. In: Humphries CJ (ed) Ontogeny and systematics. Columbia University Press, New York, pp 83–115
Blackmore S, Crane PR (1998) The evolution of apertures in the spores and pollen grains of embryophytes. In: Owens SJ, Rudall PJ (eds) Reproductive biology. Royal Botanic Gardens, Kew, pp 159–182
Blackmore S, van Helvoort H, Punt W (1984) On the terminology, origins and functions of caveate pollen in the Compositae. Rev Palaeobot Palynol 43:293–301
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 74:483–498
Blackmore S, Wortley AH, Skvarla JJ, Robinson H (2009) Evolution of pollen in the Compositae. In: Funk VA, Susanna A, Stuessy T, Bayer R (eds) Systematics, evolution and biogeography of the Compositae. IAPT, Vienna, Austria, pp 101–130
Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI (2008) Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 319:1812–1816
Collinson ME, Hemsley AR, Taylor WA (1993) Sporopollenin exhibiting colloidal organization in spore walls. Grana Suppl 1:31–39
Dickinson HG (1976) Common factors in exine deposition. In: Ferguson IK, Muller J (eds) The evolutionary significance of the exine. Academic Press, London, pp 67–89
Dickinson HG, Heslop-Harrison J (1968) Common mode of deposition for the sporopollenin of sexine and nexine. Nature 220:926–927
Dickinson HG, Potter U (1976) The development of patterning in the alveolar sexine of Cosmos bipinnatus. New Phytol 76:543–550
Dickinson HG, Sheldon JM (1986) The generation of patterning at the plasma membrane of the young microspore of Lilium. In: Blackmore S, Ferguson JK (eds) Pollen and spores: form and function. Linn. Soc. Symp. Ser. 12, pp 1–17
Dover GA (1972) The organisation and polarity of pollen mother cells of Triticum aestivum. J Cell Sci 11:699–711
El-Ghazaly G (1982) Ontogeny of pollen wall of Leontodon autumnalis (Hypochoeridinae, Compositae). Grana 21:103–113
Erdtman G (1952) Pollen morphology and plant taxonomy: angiosperms. An introduction to palynology I. Almqvist and Wiksell, Stockholm
Fitzgerald MA, Knox RB (1995) Initiation of primexine in freeze-substituted microspores of Brassica campestris. Sexual Pl Reprod 8:99–104
Florence AT (1977) Biological meaning of micellization. In: Mittal KL (ed) Micellization, solubilization, and microemulsions. Plenum Press, New York, pp 42–62
Funk VA, Bayer RJ, Keeley SC, Chan R, Watson L, Gemeinholzer B, Schilling E, Panero JL, Baldwin BG, Garcia-Jacas N, Susanna A, Jansen RK (2005) Everywhere but Antarctica: using a supertree to understand the diversity and distribution of the Compositae. Biol Skr 55:343–374
Gabarayeva NI, Grigorjeva VV (2002) Exine development in Stangeria eriopus (Stangeriaceae): ultrastructure and substructure, sporopollenin accumulation, the equivocal character of the aperture, and stereology of microspore organelles. Rev Palaeobot Palynol 122:185–218
Gabarayeva NI, Grigorjeva VV (2003) Comparative study of the pollen wall development in Illicium floridanum (Illiciaceae) and Schisandra chinensis (Schisandraceae). Taiwania 48:147–167
Gabarayeva NI, Grigorjeva VV (2004) Exine development in Encephalartos altensteinii (Cycadaceae): ultrastructure, substructure and the models of sporopollenin accumulation. Rev Palaeobot Palynol 132:175–193
Gabarayeva NI, Hemsley AR (2006) The role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol 138:121–139
Gabarayeva NI, Blackmore S, Rowley JR (2003) Observations on the experimental destruction of the pollen wall of some selected gymnosperms and angiosperms. Rev Palaeobot Palynol 124:203–226
Hemsley AR (1998) Nonlinear variation in simulated complex pattern development. J Theor Biol 192:73–79
Hemsley AR, Gabarayeva N (2007) Exine development: the importance of looking through a colloid chemistry “window”. Pl Syst Evol 263:25–49
Hemsley AR, Collinson ME, Brain APR (1992) Colloidal crystal-like structure of sporopollenin in the megaspore walls of recent Selaginella and similar fossil spores. Bot J Linn Soc 108:307–320
Hemsley AR, Griffiths PC, Matthias R, Moore SEM (2003) A model for the role of surfactants in the assembly of exine structure. Grana 42:38–42
Heslop-Harrison J (1963) An ultrastructural study of pollen wall ontogeny in Silene pendula. Grana Palynol 4:7–24
Heslop-Harrison J (1968) The pollen grain wall. Science 161:230–237
Heslop-Harrison J (1969) The origin of surface features of the pollen wall of Tagetes patula as observed by scanning electron microscopy. Cytobios 2:177–186
Heslop-Harrison J (1971) Wall pattern formation in angiosperm microsporogenesis. Symp Soc Exp Biol 25:277–300
Heslop-Harrison J (1972) Pattern in plant cell walls: morphogenesis in miniature. Proc Roy Inst Gr Brit 45:335–351
Heslop-Harrison J (1979) Pollen walls as adaptive systems. Ann Missouri Bot Gard 66:813–829
Horner HT, Pearson CB (1978) Pollen wall and aperture development in Helianthus annuus (Compositae: Heliantheae). Amer J Bot 65:293–309
Jackson RC, Skvarla JJ, Chissoe WF (2000) A unique pollen wall mutation in the family Compositae: ultrastructure and genetics. Amer J Bot 87:1571–1577
Longly B, Waterkeyn L (1979) Etude de la cytocinese. III. Les cloisonnements simultanés et successifs des microsporocytes. Cellule 73:65–80
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434
McCormick S (2004) Control of male gametophyte development. Plant Cell 16:S142–S153
Nazarova EA (1997) Karyosystematic investigation of the genus Scorzonera L. s.l. (Lactuceae, Asteraceae). Caryologia 50:239–261
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Haynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 185:7–21
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Paxson-Sowders DM, Dodrill CJ, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749
Pettitt JM (1979) Ultrastructure and cytochemistry of spore wall morphogenesis. In: Dyer AF (ed) The experimental biology of ferns. Academic Press, London, pp 211–252
Pettitt JM, Jermy AC (1974) The surface coats on spores. Biol J Linn Soc 6:245–257
Rowley JR (1971) Implications on the nature of sporopollenin based upon pollen development. In: Brooks J, Grant PR, Muir M, van Gijzel P, Shaw G (eds) Sporopollenin. Academic Press, London, pp 174–219
Rowley JR (1973) Formation of pollen exine bacules and microchannels on a glycocalyx. Grana 13:129–138
Rowley JR (1980) The origin, ontogeny and evolution of the exine. Proceedings of the IVth International Palynological Conference, Lucknow, 1976–1977. 1:126–136
Rowley JR (1990) The fundamental structure of the exine. Pl Syst Evol (Suppl. 5):13–29
Rowley JR, Claugher D (1991) Receptor-independent sporopollenin. Bot Acta 104:316–323
Rowley JR, Dahl AO (1977) Pollen development in Artemisa vulgaris with special reference to glycoclayx material (1). Pollen Spores 14:169–284
Rowley JR, Prijianto B (1977) Selective destruction of the exine of pollen grains. Geophytol 7:1–23
Rowley JR, Skvarla JJ (1975) The glycocalyx and initiation of exine spinules on microspores of Canna. Amer J Bot 62:479–485
Rowley JR, Skvarla JJ (1993) Exine receptors. Grana 2:21–25
Rowley JR, Skvarla JJ (2007) Pollen development in Epilobium (Onagraceae): from microspore mitosis to formation of the intine. Grana 46:130–139
Rowley JR, Southworth D (1967) Deposition of sporopollenin on lamellae of unit membrane dimensions. Nature 213:703–704
Rowley JR, Dahl AO, Rowley JS (1981) Substructure in exines of Artemisia vulgaris (Asteraceae). Rev Palaeobot Palynol 35:1–38
Rowley JR, Claugher D, Skvarla JJ (1999a) Structure of the exine in Artemisia vulgaris (Asteraeae): a review. Taiwania 44:1–21
Rowley JR, Skvarla JJ, Gabarayeva NI (1999b) Exine development in Borago (Boraginaceae): 2. Free microspore stages. Taiwania 44:212–229
Rowley JR, Skvarla JJ, Walles B (1999c) Microsporogenesis in Pinus sylvestris.—VII. Exine expansion and tapetal development. Taiwania 44:325–344
Scott RJ (1994) Pollen exine—the sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead MA (eds) Society for experimental biology seminar series 55: molecular and cellular aspects of plant reproduction. Cambridge University Press, Cambridge, pp 49–81
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall in Lilium henryi. J Cell Sci 63:191–208
Skvarla JJ, Larson DA (1965) An electron microscopic study of pollen morphology in the Compositae with special reference to the Ambrosiinae. Grana 6:210–269
Skvarla JJ, Turner BL (1966) Systematic implications from electron microscopic studies of Compositae pollen—a review. Ann Missouri Bot Gard 53:220–256
Skvarla JJ, Turner BL, Patel VC, Tomb AS (1977) Pollen morphology in the Compositae and in morphologically related families. In: Heywood VH, Harborne JB, Turner BL (eds) The biology and chemistry of the Compositae. Academic Press, London, pp 141–248
Skvarla JJ, Rowley JR, Chissoe WF, Strout G (2001) Incomplete exine development in aborted pollen of Eupatorium serotinum Michx. (Compositae: Eupatorieae). Taiwania 46:103–113
Southworth D (1974) Solubility of pollen exines. Amer J Bot 61:36–44
Southworth D (1983) Exine development in Gerbera jamesonii (Asteraceae: Mutisieae). Amer J Bot 70:1038–1047
Southworth D (1986) Substructural organization of pollen exines. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 61–69
Southworth D, Jernstedt JA (1995) Pollen exine development precedes microtubule rearrangement in Vigna unguiculata (Fabaceae): a model for pollen wall patterning. Protoplasma 187:79–87
Stix E (1960) Pollenmorphologische Untersuchungen an Compositen. Grana 2:41–104
Takahashi M (1989) Development of the echinate pollen wall in Farfugium japonicum (Compositae: Senecioneae). Bot Mag (Tokyo) 102:219–234
Thomson DA (1917) On growth and form. Cambridge University Press, Cambridge
Tomb AS, Larson DA, Skvarla JJ (1974) Pollen morphology and detailed structure of family Compositae, tribe Cichorieae. I. Subtribe Stephanomeriinae. Amer J Bot 61:486–498
Turner BL (1977) Summary of the biology of the Compositae. In: Heywood VH, Harborne JB, Turner BL (eds) The biology and chemistry of the Compositae. Academic Press, London, pp 1105–1118
Varotto S, Parrini P, Mariani P (1996) Pollen ontogeny in Cichorium intybus L. Grana 35:154–161
Wagenitz G (1955) Pollenmorphologie und Systematik in der Gattung Centaurea L. s.l. Flora 142:213–279
Wagenitz G (1976) Systematics and phylogeny of the Compositae. Pl Syst Evol 125:29–46
Wodehouse RP (1930) Pollen grains in the identification and classification of plants. V. Haplopappus and other Astereae: the origin of their furrow configurations. Amer J Bot 16:297–313
Wodehouse RP (1931) The origin of the six-furrowed configuration of Dahlia pollen grains. Bull Torrey Bot Club 57:371–380
Wodehouse RP (1935) Pollen grains: their structure, identification and significance in science and medicine. McGraw–Hill Book Company, Inc., New York
Wortley AH, Blackmore S, Skvarla JJ (2009) Bibliography of pollen literature in the Compositae. In: Funk VA, Susanna A, Stuessy T, Bayer R (eds) Systematics, Evolution and biogeography of the Compositae. IAPT, Vienna, Austria, pp 807–867
Acknowledgments
The authors are grateful to Donald Claugher and Susan H. Barnes for helping to shape our ideas about pollen development and exine ontogeny. This paper was first conceived as part of a Festschrift in honour of Gerhard Wagenitz; we thank the organisers and Professor Wagenitz himself for their inspiration.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of Donald Claugher.
Rights and permissions
About this article
Cite this article
Blackmore, S., Wortley, A.H., Skvarla, J.J. et al. Developmental origins of structural diversity in pollen walls of Compositae. Plant Syst Evol 284, 17–32 (2010). https://doi.org/10.1007/s00606-009-0232-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-009-0232-2