Abstract
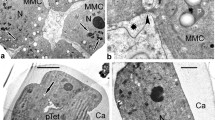
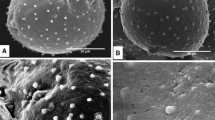
The structure of Cupressus arizonica pollen at different degrees of hydration was examined by using cytochemical staining and light (LM) and scanning electron (SEM) microscopy. Most pollen grains are inaperturate and a minority are provided with an operculate pore enveloped by a concave annulus. Intine consists of: 1) a thin polysaccharidic outer layer, 2) a large polysaccharidic middle layer that is spongy and bordered by a mesh of large and branched fibrils, and 3) an inner cellulosic thick layer with callose concentrated on the inner side, which forms a shell around the protoplast. The protoplast is egg-shaped with PAS positive cytoplasm and prominent nucleus. Exine splits during hydration and is cast off according to three major steps: 1) the split opens like a mouth and the underlying intine is expelled by swelling like a balloon, 2) the protoplast enveloped by the inner intine is sucked in the outgrowing side, and 3) the backside of the intine gets rid of the exine shell. In water containing salts, exine is rapidly released and the middle intine may expand up to break the outer layer, with disgregation of the spongy material and release of the intine shell including the protoplast. In water lacking salts, the sporoderm hydration and breaking are negatively influenced by the population effect. Pollen when air dried after the exine release become completely flat owing to disappearance of the middle intine layer which may be restored by dipping pollen in water. The results are discussed in relation to the functional potentialities of the sporoderm.
Similar content being viewed by others
References
Bortenschlager S (1990). Aspects of pollen morphology in the Cupressaceae. Grana 29: 129–137
Canini A, Gioninazzi J, Iacovacci P, Pini C and Grilli Caiola M (2004). Localisation of a carbohydrate epitope recognised by human IgE in pollen of Cupressaceae. J Pl Res 117: 147–153
Charpin D, Calleja M, Lahoz C, Pichot C and Waisel Y (2005). Allergy to cypress pollen. Allergy 60: 293–301
Chichiriccò G (2006). Calcium in the micropylar secretion and receptivity of explanted Crocus ovules to intra- and interspecific pollen. Pl Syst Evol 262: 89–96
Coleman AW and Goff LJ (1985). Application of fluorochromes to pollen biology. 1. Mithramycin-8–4,6-diamino-2-phenylindole (DAPI) as vital stain and for quantitation of nuclear DNA. Stain Technol 60: 145–154
Duhoux E (1982). Mechanism of exine rupture in hydrated taxoid type of pollen. Grana 21: 1–7
Erdtman G (1960). The acetolysis method. A revised description. Svensk Bot Tidskr 54: 561–564
Fernando D, Lazzaro MD and Owens JN (2005). Growth and development of conifer pollen tubes. Sex Pl Reprod 18: 149–162
Grilli Caiola M, Travaglini A and Giuliano M (2000). Palynological study of Cupressus sempervirens L. var. pyramidalis and var. horizontalis. Pl Biosys 134: 99–109
Heslop- Harrison J, Heslop-Harrison Y (1985) Germination of stress-to lerovat Eucalyptus pollen. J Cell Sci 73: 135–157
Heslop-Harrison J and Heslop-Harrison Y (1991). Structural and functional variation in pollen intines. In: Blackmore S, Barmes SH (eds) Pollen Spores. Syst. Ass. Special Vol 44: 331–343
Hughes J and McCully ME (1975). The use of an optical brightener in the study of plant structure. Stain Technol 50: 319–329
Johansen DA (1940). Plant microtechnique. McGraw-Hill, New York
Kurmann MH (1994). Pollen morphology and ultrastructure in the Cupressaceae. Acta Bot Gallica 141: 141–147
Mori B and Bellani LM (1996). Differential staining for cellulosic and modified plant cell walls. Biotech Histochem 71: 71–72
Mothes N, Horak F and Valenta R (2004). Transition from a botanical to a molecular classification in tree pollen allergy: Implications for diagnosis and therapy. Int Arch Allergy Immunol 135: 357–373
O’Brien TP and McCully ME (1981). The study of plant structure. Principles and selected methods. Thermarcarphi pty. Ltd, Melbourne
Osborn JM, El Ghazaly G and Cooper RL (2001). Development of exineless pollen wall in Callitriche truncata (Callitrichaceae) and the evolution of underwater pollination. Pl Syst Evol 228: 81–87
Pacini E, Franchi G and Ripaccioli M (1999). Ripe pollen structure and histochemistry of some gymnosperms. Pl Syst Evol 217: 81–99
Pardi ML, Viegi L, Cela Renzoni G, Franchi G and Pacini E (1996). Effects of different pH values on insoluble polysaccharide content of germinating pollen of Pinus pinea and Pinus pinaster. Grana 35: 240–247
Suárez-Cervera M, Takahashi Y, Vega-Maray A and Seoane-Camba JA (2003). Immunocytochemical localization of Cry j 1, the major allergen of Cryptomeria japonica (Taxodiaceae) in Cupressus arizonica and Cupressus sempervirens (Cupressaceae) pollen grains. Sex Pl Reprod 16: 9–15
Van Campo M (1953) Recherches sur la phylogénie des Cupressacées d’après leurs grains de pollen. Tran. Lab. Forest. Toulouse 2: 1–20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chichiriccò, G., Pacini, E. Cupressus arizonica pollen wall zonation and in vitro hydration. Plant Syst Evol 270, 231–242 (2008). https://doi.org/10.1007/s00606-007-0610-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-007-0610-6