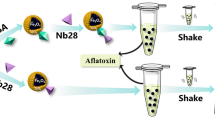
Abstract
It has been observed that polyvalent metal ions can mediate the adsorption of DNA on polydopamine (PDA) surfaces. Exploiting this, we used two divalent metal ions (Mg2+ or Ca2+) to promote the adsorption of fluorescence-labelled ochratoxin A (OTA) aptamers on PDA-coated magnetic nanoparticles (Fe3O4@PDA). Based on the different adsorption affinities of free aptamers and OTA-bound aptamers, a facile assay method was established for OTA detection. The aptamers adsorbed on Fe3O4@PDA were removed via simple magnetic separation, and the remaining aptamers in the supernatant exhibited a positive correlation with the OTA concentration. The concentrations of Mg2+ and Ca2+ were finely tuned to attain the optimal adsorption affinity and sensitivity for OTA detection. In addition, other factors, including the Fe3O4@PDA dosage, pH, mixing order, and incubation time, were studied. Finally, under optimized conditions, a detection limit (3σ/s) of 1.26 ng/mL was achieved for OTA. Real samples of spiked red wine were analysed with this aptamer-based method. This is the first report of regulating aptamer adsorption on the PDA surface with polyvalent metal ions for OTA detection. By changing the aptamers, the method can be easily extended to other target analytes.
Graphical abstract






Similar content being viewed by others
Data availability
Data will be made available on request.
References
Yu HX, Alkhamis O, Canoura J, Liu YZ, Xiao Y (2021) Advances and challenges in small-molecule DNA aptamer isolation, characterization, and sensor development. Angew Chem Int Ed 60:16800–16823. https://doi.org/10.1002/anie.202008663
Dunn MR, Jimenez RM, Chaput JC (2017) Analysis of aptamer discovery and technology. Nat Rev Chem 1:0076. https://doi.org/10.1038/s41570-017-0076
Wu Y, Belmonte I, Sykes KS, Xiao Y, White RJ (2019) Perspective on the future role of aptamers in analytical chemistry. Anal Chem 91:15335–15344. https://doi.org/10.1021/acs.analchem.9b03853
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430. https://doi.org/10.1126/science.1147241
Hong S, Na YS, Choi S, Song IT, Kim WY, Lee H (2012) Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv Funct Mater 22:4711–4717. https://doi.org/10.1002/adfm.201201156
Zandieh M, Hagar BM, Liu JW (2020) Interfacing DNA and polydopamine nanoparticles and its applications. Part Part Syst Charact 37:2000208. https://doi.org/10.1002/ppsc.202000208
Meng YC, Liu P, Zhou WH, Ding JS, Liu JW (2018) Bioorthogonal DNA adsorption on polydopamine nanoparticles mediated by metal coordination for highly robust sensing in serum and living cells. ACS Nano 12:9070–9080. https://doi.org/10.1021/acsnano.8b03019
Zandieh M, Liu JW (2020) Transition metal-mediated DNA adsorption on polydopamine nanoparticles. Langmuir 36:3260–3267. https://doi.org/10.1021/acs.langmuir.0c00046
Zandieh M, Liu JW (2021) Metal-doped polydopamine nanoparticles for highly robust and efficient DNA adsorption and sensing. Langmuir 37:8953–8960. https://doi.org/10.1021/acs.langmuir.1c00783
Kushalkar MP, Liu BW, Liu JW (2020) Promoting DNA adsorption by acids and polyvalent cations: beyond charge screening. Langmuir 36:11183–11195. https://doi.org/10.1021/acs.langmuir.0c02122
Qiang WB, Li W, Li XQ, Chen X, Xu DK (2014) Bioinspired polydopamine nanospheres: a superquencher for fluorescence sensing of biomolecules. Chem Sci 5:3018–3024. https://doi.org/10.1039/c4sc00085d
Qiang WB, Hu HT, Sun L, Li H, Xu DK (2015) Aptamer/polydopamine nanospheres nanocomplex for in situ molecular sensing in living cells. Anal Chem 87:12190–12196. https://doi.org/10.1021/acs.analchem.5b03075
Guo T, Wang CC, Zhou HY, Zhang YH, Ma L, Wang S (2021) A facile aptasensor based on polydopamine nanospheres for high-sensitivity sensing of T-2 toxin. Anal Methods 13:2654–2658. https://doi.org/10.1039/d1ay00642h
Liu Q, Pu ZH, Asiri AM, Al-Youbi AO, Sun XP (2014) Polydopamine nanospheres: A biopolymer-based fluorescent sensing platform for DNA detection. Sens Actuators B Chem 191:567–571. https://doi.org/10.1016/j.snb.2013.10.050
Liu XJ, Lin BX, Yu Y, Cao YJ, Guo ML (2018) A multifunctional probe based on the use of labeled aptamer and magnetic nanoparticles for fluorometric determination of adenosine 5’-triphosphate. Microchim Acta 185:243. https://doi.org/10.1007/s00604-018-2774-x
Sun YJ, Wang CC, Tang LN, Zhang YL, Zhang GJ (2021) Magnetic-enhanced fluorescence sensing of tumor miRNA by combination of MNPs@PDA with duplex specific nuclease. RSC Adv 11:2968–2975. https://doi.org/10.1039/d0ra09237a
Cao XD, Zhang KR, Yan WW, Xia ZH, He SD, Xu X, Ye YK, Wei ZJ, Liu SQ (2020) Calcium ion assisted fluorescence determination of microRNA-167 using carbon dots-labeled probe DNA and polydopamine-coated Fe3O4 nanoparticles. Microchim Acta 187:212. https://doi.org/10.1007/s00604-020-4209-8
Jiang HY, Xia Q, Liu DJ, Ling K (2020) Calcium-cation-doped polydopamine-modified 2D black phosphorus nanosheets as a robust platform for sensitive and specific biomolecule sensing. Anal Chim Acta 1121:1–10. https://doi.org/10.1016/j.aca.2020.04.072
Alhamoud Y, Yang DT, Fiati Kenston SS, Liu GZ, Liu LY, Zhou HB, Ahmed F, Zhao JS (2019) Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens Bioelectron 141:111418. https://doi.org/10.1016/j.bios.2019.111418
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res 51:61–99. https://doi.org/10.1002/mnfr.200600137
Meira DI, Barbosa AI, Borges J, Reis RL, Correlo VM, Vaz F (2023) Recent advances in nanomaterial-based optical biosensors for food safety applications: ochratoxin-A detection, as case study. Crit Rev Food Sci Nutr 1–43. https://doi.org/10.1080/10408398.2023.2168248
Cruz-Aguado JA, Penner G (2008) Determination of ochratoxin A with a DNA aptamer. J Agric Food Chem 56:10456–10461. https://doi.org/10.1021/jf801957h
Geng X, Zhang DP, Wang HL, Zhao Q (2013) Screening interaction between ochratoxin A and aptamers by fluorescence anisotropy approach. Anal Bioanal Chem 405:2443–2449. https://doi.org/10.1007/s00216-013-6736-1
Xu GH, Zhao JJ, Yu H, Wang C, Huang YY, Zhao Q, Zhou X, Li CG, Liu ML (2022) Structural insights into the mechanism of high-affinity binding of ochratoxin A by a DNA aptamer. J Am Chem Soc 144:7731–7740. https://doi.org/10.1021/jacs.2c00478
Liu J, Sun ZK, Deng YH, Zou Y, Li CY, Guo XH, Xiong LQ, Gao Y, Li FY, Zhao DY (2009) Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew Chem Int Ed 48:5875–5879. https://doi.org/10.1002/anie.200901566
Yang C, Lates V, Prieto-Simon B, Marty JL, Yang XR (2012) Aptamer-DNAzyme hairpins for biosensing of ochratoxin A. Biosens Bioelectron 32:208–212. https://doi.org/10.1016/j.bios.2011.12.011
Yang C, Lates V, Prieto-Simon B, Marty JL, Yang XR (2013) Rapid high-throughput analysis of ochratoxin A by the self-assembly of DNAzyme-aptamer conjugates in wine. Talanta 116:520–526. https://doi.org/10.1016/j.talanta.2013.07.011
Sheng DH, Ying XT, Li R, Cheng SY, Zhang C, Dong W, Pan XH (2022) Polydopamine-mediated modification of ZIF-8 onto magnetic nanoparticles for enhanced tetracycline adsorption from wastewater. Chemosphere 308:136249. https://doi.org/10.1016/j.chemosphere.2022.136249
Pan XH, Cheng SY, Su T, Zuo GC, Zhang C, Wu LP, Jiao YZ, Dong W (2019) Poly (2-hydroxypropylene imines) functionalized magnetic polydopamine nanoparticles for high-efficiency DNA isolation. Appl Surf Sci 498:143888. https://doi.org/10.1016/j.apsusc.2019.143888
Yang W, Liu C, Chen Y (2018) Stability of polydopamine coatings on gold substrates inspected by surface plasmon resonance imaging. Langmuir 34:3565–3571. https://doi.org/10.1021/acs.langmuir.7b03143
Yang C, Wang Y, Marty J-L, Yang XR (2011) Aptamer-based colorimetric biosensing of ochratoxin A using unmodified gold nanoparticles indicator. Biosens and Bioelectron 26:2724–2727. https://doi.org/10.1016/j.bios.2010.09.032
Duan N, Ren KX, Song MQ, Zhang XY, Wang ZP, Jia F, Wu SJ (2023) A dual-donor FRET based aptamer sensor for simultaneous determination of histamine and tyramine in fishes. Microchem J 191:108801. https://doi.org/10.1016/j.microc.2023.108801
He Y, Lin Y, Tang HW, Pang DW (2012) A graphene oxide-based fluorescent aptasensor for the turn-on detection of epithelial tumor marker mucin 1. Nanoscale 4:2054–2059. https://doi.org/10.1039/c2nr12061e
Funding
This work was supported by the CAS scientific instruments and equipment development program (Grant: YJKYYQ20200008) and the AI S&T Program of Yulin Branch, Dalian National Laboratory for Clean Energy, CAS (Grant No. DNL-YL A202203).
Author information
Authors and Affiliations
Contributions
Wei Yang: Conceptualization, Methodology, Validation, Investigation, Visualization, Writing-Original Draft. Lanxiu Ni: Investigation, Validation. Mingzhen Zhu: Methodology, Investigation. Xiaobo Zhang: Methodology, Investigation. Liang Feng: Supervision, Writing-Review&Editing, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
This research did not involve human or animal samples.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, W., Ni, L., Zhu, M. et al. Mg2+- or Ca2+-regulated aptamer adsorption on polydopamine-coated magnetic nanoparticles for fluorescence detection of ochratoxin A. Microchim Acta 191, 157 (2024). https://doi.org/10.1007/s00604-024-06252-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06252-0