Abstract
The intricate tapestry of biomarkers, including proteins, lipids, carbohydrates, vesicles, and nucleic acids within sweat, exhibits a profound correlation with the ones in the bloodstream. The facile extraction of samples from sweat glands has recently positioned sweat sampling at the forefront of non-invasive health monitoring and diagnostics. While extant platforms for sweat analysis exist, the imperative for portability, cost-effectiveness, ease of manufacture, and expeditious turnaround underscores the necessity for parameters that transcend conventional considerations. In this regard, 3D printed microfluidic devices emerge as promising systems, offering a harmonious fusion of attributes such as multifunctional integration, flexibility, biocompatibility, a controlled closed environment, and a minimal requisite analyte volume—features that leverage their prominence in the realm of sweat analysis. However, formidable challenges, including high throughput demands, chemical interactions intrinsic to the printing materials, size constraints, and durability concerns, beset the landscape of 3D printed microfluidic devices. Within this paradigm, we expound upon the foundational aspects of 3D printed microfluidic devices and proffer a distinctive perspective by delving into the computational study of printing materials utilizing density functional theory (DFT) and molecular dynamics (MD) methodologies. This multifaceted approach serves manifold purposes: (i) understanding the complexity of microfluidic systems, (ii) facilitating comprehensive analyses, (iii) saving both cost and time, (iv) improving design optimization, and (v) augmenting resolution. In a nutshell, the allure of 3D printing lies in its capacity for affordable and expeditious production, offering seamless integration of diverse components into microfluidic devices—a testament to their inherent utility in the domain of sweat analysis. The synergistic fusion of computational assessment methodologies with materials science not only optimizes analysis and production processes, but also expedites their widespread accessibility, ensuring continuous biomarker monitoring from sweat for end-users.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, humanity has fervently embraced technologies that empower individuals to scrutinize their health status [1]. Particularly, glucose sensors stand as vanguards in this wave of transformative technological progress [2]. Traditionally, the analysis of target molecules was confined to laboratory settings before becoming accessible to the end-users. However, with the evolution of biosensor technologies, these analyses can now be conveniently conducted in domestic environments, thereby effecting significant savings in both cost and time [3, 4]. Analysis of sweat, a complex biological fluid containing various analytes, is crucial for tracking health conditions [5, 6] such as drug and alcohol monitoring [7], temperature regulation [8], infectious diseases [9], glucose levels [10], and hydration levels [11] (Fig. 1).
During the metamorphosis of bulky biosensing systems into their portable versions, microfluidic systems have taken a crucial role since they offer a broader range of miniaturized total chemical analysis systems based on the manipulation of fluidic devices in micro/nanoscale volumes [12, 13]. Investigations of microfluidic devices and their fabrication with micromechanics technology back in the 1970s first launched with gas chromatograph and inkjet printer nozzles, then continued with flow sensors and valves to complex microfluidic systems for chemical and biological analysis. Various fabrication methods have been employed for microfluidic device fabrication and continually developed [14, 15]. The availability of material, environmental impact, device dimension, cost, and rapid fabrication process are critical parameters affecting overall device performance and wide application [16]. Among them, 3D printing technologies are emerging advancements with varied techniques, including stereolithography (SLA) [17], two-photon polymerization (2PP) [18], fused deposition modeling (FDM) [19], digital light processing (DLP) [20], and PolyJet [21] techniques which are adaptable methods for microfluidic device fabrication. The materials selection depends on which 3D printing techniques are employed, including plastic (acrylonitrile, 1,3-butadiene, and styrene (ABS) [22], poly methyl methacrylate (PMMA) [23], and poly lactic acid (PLA) [24]), metal (titanium [25] and Inconel 625 [26]), and composite materials (carbon fiber [27] and fiberglass [28]). Even though many of these materials cannot be used directly, they are added to the printing material to increase the materials’ heat capacity or mechanical strength [29, 30].
In addition, one of the strongest points of these 3D printed microfluidic devices, which can be produced quickly and at a low-cost, is that they can be customized to the person’s requirements. Moreover, the properties of printing materials, including flexibility [31], optical transmittance [32], and heat and mechanical resistance [29, 30], can be altered and produced by including additives or adjusting device settings (such as exposure time and resolution) in the direction of interest. In addition to improving the quality of these materials, properties such as pore width, flexibility, and mechanical strength of materials are made possible by analyzing them at the molecular level, owing to computational techniques such as DFT [33, 34]. Moreover, the sensor system (tattoo [35] and pH monitoring [36]) to be developed depending on the target molecule can be included in these microfluidic systems at the end of the day. By adding electronic circuits to these systems, information flow can be instantly transferred to devices such as smartphones via NFC, Wi-Fi, or Bluetooth [37, 38].
Focusing on the adaptation of health monitoring systems for daily use, low-cost, short turnaround time, and ease of use are pivotal parameters for the end-users. Therefore, 3D printed microfluidic devices for sweat analysis would be a niche for this manner. Moreover, blood drawing requires invasive methods such as needles. Since sweat is on the body’s surface, it does not require using any needles or other invasive methods [39], and this is an appealing point for patients, newborns, children, and those who are having difficulties in blood sampling [40]. Briefly, sweat is a rich source of biomarkers including ions (sodium (Na+), potassium (K+), and chloride (Cl−)), metabolites (lactate, glucose, and urea), hormones (cortisol and testosterone), metals (arsenic (As) and lead (Pb)), and enzymes (amylase and creatine kinase) in which are secreted from the sweat glands in the dermis and secreted through the dermal duct from dermis to the uppermost layer of the epidermis to the skin [41]. Changing conditions of the body fluids are also reflected to the sweat since the sweat secretion is an ultimate route of channeling toxins and disposed molecules out of the body. This rich mixture of components makes sweat as an ultimate source of biomarker detection related to different diseases [42, 43]. Moreover, continuous monitoring can be implemented by constantly monitoring sweating. To exemplify, Nyein et al. managed to continuously and autonomously monitor the body’s pH, Cl−, and levodopa levels by developing a wearable patch. By this means, it was possible to watch stress situations, activities, and findings associated with Parkinson’s disease instantly [44]. Many different studies have revealed that the amounts of glucose and lactate in blood are strongly related to the levels of molecules in sweat [40]. To simply put the combination of the ability to perform multiple analyses using sweat and the advantages of those mentioned above, 3D printed microfluidic devices help to put forward a promising health monitoring system.
This review aspires to undertake a thorough exploration of the application of 3D printed microfluidic systems integrated with biosensors for detecting biomarkers in sweat. Moreover, our endeavor extends to bridging the divide between these platforms and computational perspectives by elucidating select computational studies focused on the materials integral to 3D printing for advancing microfluidic systems for health monitoring. In this regard, computational strategies would enhance comprehension of the intricacies inherent in microfluidic systems, facilitate in-depth and comprehensive analyses, concurrently economize both cost and time, refine the optimization of design elements, and bolster the resolution. The studies mentioned below are directed towards propelling significant strides in enhancing the manufacturability and adaptability of 3D-printed microfluidic systems, with the ultimate aim of meeting the discerning needs and expectations of the end-users.
3D printing devices for fabricating microfluidic systems
Conventional microfluidic fabrication methods heavily depend on expensive and sophisticated cleanroom facilities, including soft photolithography steps that are time-consuming and tedious processes [45]. On the other hand, 3D printing, as a revolutionary additive manufacturing technology, provides a simplified, facile, and accessible method for fabricating microfluidic devices in one or two steps [46]. Contrary to the traditional methods, 3D printing minimizes the labor of the fabrication process, eliminating cleanroom setups and reducing the time required to fabricate microfluidic systems. 3D printing in microfluidics allows complex, robust, and intricate structures. 3D printing methods (SLA, 2PP, FDM, DLP, and PolyJet) offer the rapid production [47] of microfluidic devices, forming complex channel structures, precise control over dimensions, and several materials, including tailored resins for diverse microfluidic applications [48]. Various microfluidic devices with different functionalities can be fabricated, including 3D microfluidics for cell culture [49], biosensing [50], drug delivery, inertial microfluidics [51], micromixers [16], and droplet-based microfluidics [52].
Briefly, SLA employs a focused UV laser to selectively cure layers of liquid photopolymer resin to build the desired structures by controlling the positions of the laser focus, layer-by-layer, repeatedly and gradually. SLA provides delicate, intricate structures with high resolution and smooth surfaces, but it is limited to a slow speed due to single-point laser operation [53]. SLA can be operated either in free (bath) or constrained (bat) configurations, as demonstrated in Fig. 2A, B. In the free surface method, a mobile build platform is submerged in a tank with photo-curable resin, while the constrained surface method utilizes a platform above the resin tank cured with UV light. DLP works similarly to SLA, yet a light source is utilized instead of a UV source to cure photopolymer resin. It is faster than SLA due to the simultaneous curing of all layers at once. In the 2PP technique, a femtosecond laser is employed to cure photopolymers similar to SLA. However, it can fabricate a volume structure without a layers-by-layer technique due to a photoinitiator’s absorption of two photons. The representation of SLA that applies a one-photon laser and two-photon laser of 2PP is depicted in Fig. 2C. Although high resolution is achieved and many materials could be adaptable, it is a slower technique than SLA and FDM.
Schematic illustrations of 3D printing techniques. (A) free and (B) constrained configurations of SLA 3D printing systems. Reprinted with permission [55]. Copyright 2022, Royal Society of Chemistry. (C) one-photon laser (i) and two-photon laser (ii) absorption. Reprinted with permission [67]. Copyright 2006, Elsevier. (D) PolyJet 3D printing system [54]. (E) FDM 3D printing system. Reprinted with permission [54]. Copyright 2016, Royal Society of Chemistry. (F) Layer-by-layer printing. Reprinted with permission [58]. Copyright 2020, Science Advances. (G) Comparison of roughness and resolution in microfluidics fabricated by SLA-DLP (i), PolyJet (ii), and FDM (iii) 3D printing. Reprinted with permission [56]. Copyright 2017, American Chemical Society (ACS)
As a second method, PolyJet printing operates by jetting a liquid photosensitive polymer ink to deposit layers and curing with UV light to obtain desired structures. This method has the potential for mass production and enables complex multi-material fabrication, including rigid and flexible forms. Employing diverse materials enables the creation of objects with different properties, like soft and hard plastics, elastomers, and different colors. Its high resolution and multi-material properties make more attractive for the applications of microfluidic devices. However, the tedious post-process of cleaning the sacrificial layer and the cost are the main limitations to boosting its applicability [54].
FDM technology is commonly used for 3D printing; a thermoplastic is melted and extruded through a heated nozzle and deposited layer by layer to build a microfluidic structure [55]. Despite the fact that it is widely used with many thermoplastic polymers, the resolution and strength of structures are low due to incomplete layer fusion. FDM is cost-effective and straightforward; however, it is limited to low precision and pore formation compared to SLA and DLP techniques.
The working principles of SLA, PolyJet, FDM, and layer-by-layer 3D printing technologies, along with their respective components, are depicted in Fig. 2. A comprehensive study is illustrated in Fig. 2G, which evaluates microfluidic performance printed by SLA-DLP, FDM, and PolyJet 3D printing techniques. It has been shown that SLA-DLP offers less roughness, making it more suitable for precise flow control. On the other hand, SLA-DLP and PolyJet provide high resolution according to FDM techniques [56]. Although 3D microfluidic-based printing is a promising fabrication method, it has yet to reach the level of resolution required by lithographic techniques. 3D printing technology for microfluidic devices with high precision is in the developmental stage, and further advancements in materials science, optimization processes, and printing techniques are necessary to expand its applicability.
Materials for 3D printed chips
Various thermoplastic polymers, photopolymers, and elastomers can be implemented for 3D printing microfluidic devices [57, 58]. The choice of materials holds significant importance within microfluidics, influencing critical aspects such as resolution, mechanical strength, transparency, biocompatibility, surface quality, and manipulation of fluid flow. Each of these parameters plays a pivotal role that impacts the overall functionality and performance of the microfluidic application. As an example, polydimethylsiloxane (PDMS) is a widely used elastomer in microfluidics due to its inertness, robust structure, and transparency, which is conventionally utilized with soft lithography for microfluidic fabrication [55]. 3D printing enables the fabrication of PDMS either directly or as a mold [59]. The 3D printing for PDMS as a mold is reported down to a 10-µm feature resolution [60]. Fabrication of microfluidic devices employs a series of lithography techniques. A photomask is generated initially by soft lithography to transfer the pattern to SU8 spin-coated silicon wafers. Following the pouring and peeling of PDMS, the glass substrate is encapsulated with PDMS via oxygen plasma bonding. In contrast, 3D presents a simple approach and eliminates multistep fabricating a PDMS as a mold or direct fabrication of a complete microfluidic device while retaining its oxygen permeability and biocompatibility [53]. The PDMS mold and the whole microfluidic device can be fabricated using SLA, DLP, FDA, and PolyJet 3D printing techniques.
Thermoplastic is a sub-division of polymers that soften when heated and solidify when cooled. Thermoplastics are reprocessable and do not undergo irreversible changes, unlike thermosetting polymers, which allow them to be melted, reshaped, and solidified many times while retaining their properties. Almost all thermoplastic can be printed by FDM technology. There are numerous commercial thermoplastics that can be employed in 3D printing, including PMMA, PLA [61], polycarbonate (PC) [62], ABS [63], polypropylene (PP), and polyethylene terephthalate glycol (PETg). Among them, ABS and PLA polymers are widely utilized for robust microfluidic systems due to their durability and mechanical strength. Besides direct printing of microfluidic devices, PLA and ABS-based molds can be printed via FDM printing [64].
Photopolymerization is a chemical reaction triggered by photosensitive material when exposed to light, generally a UV or visible light wavelength. Hence, it undergoes a process where the molecules within polymer material react and form large molecule structures. Acrylates, epoxides, and urethanes are mainly photopolymers that have been used, and they are prone to swelling in some solvents, which inhibits their wide range of applications [65]. In addition, SLA, DLP, and 2PP, PolyJet technologies, utilize photopolymers [66].
Sweat analysis through biosensors integrated with 3D printed chips
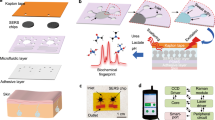
The ongoing literature effort has turned into a few designs that could integrate 3D printed microfluidic platforms as sweat analysis platforms combined with in-device biosensors. In a pioneering study, researchers demonstrated one of the first examples of 3D printed microfluidic chips [68]. The molten-syrup-based 3D printing technique used in this study is an eco-friendly and low-cost alternative to plastic-based 3D printing materials. Positive molds created with molten-syrup printing were later complemented with PDMS and additive layers with embedded electrodes to form the final skin mounting device for sweat collection and analysis. Integrated electrochemical sensor to the system with electrodes resulted in the comparative readout of sweat pH values compared with a commercial pH-meter in a narrow measurement range and real-time fashion. In another study, a 3D printed microfluidic sampling module with a flexible and multiplex sensor module patch from an integrated circuit is demonstrated [69]. Overall, the design in this study is capable of monitoring multiple electrolytes (Na+, K+, and Ca2+) from non-invasively collected sweat samples. As a result, all three target ions could be detected between 0.1 and 100 mM concentration using the silver 3D printed electrode including ion-selective membrane. In order to analyze more indicators from sweat, Yang et al. demonstrated a multiplexed sweat analysis module based on spectroscopic measurement [70]. This study initially investigates the properties of flexible 3D printing resins by printing a microfluidic design with sweat containers and reaction chambers in order to achieve an ideal measurement chamber for the optic components. Next, the reaction chambers were filled with either colorimetric assays for the concentration determination of Cu2+, Cl−, and pH values or a fluorometric reaction for glucose concentration detection (Fig. 3A). The resulting system, which is attached with a medical-grade adhesive to the skin, shows compatible results in human trials with laboratory results on a scale of 0–3 ppm for copper, 0–160 mM for Cl, pH 4.0 to pH 9.0, and 0–50 µM for glucose.
(A) Fabrication steps of 3D printed microfluidic sweat analysis device is depicted. (B) The layers of the device, (C) the analysis methods for the target analytes, (D−F) internal structure of the device with different dyes, and (G) the completed device are presented. Reprinted with permission [70]. Copyright 2023, Royal Society of Chemistry
Additionally, 3D printing could be used not only to design microfluidic systems, but also measurement chambers for sweat collection studies. In a different study, a smartphone-based lactate measurement system was developed based on lactate oxidize reaction and detecting hydrogen peroxide output with horseradish peroxidase (HRP) luminometric assay. The overall system was composed of a mini cartridge that contains the reactions, a dark box to contain light output generated by HRP-luminol reaction, and a universal holder for smartphones [71]. The designed device is capable of measuring lactate levels in sweat and saliva with the millimolar level per liter in both types of samples. Apart from being a production platform for microfluidic chips, 3D printing could also assist microfluidic system production for sweat analysis by being a low-cost and high-volume platform for mold production. In Nah et al. (2021) study, scientists utilized this advantage of 3D printing by designing a flexible microfluidic system based on PDMS reaction chambers and Ti3C2Tx MXene-loaded laser-burned graphene as flexible electrode material on flexible polyimide (PI) film, for later also to be transferred to PDMS [72].
Considering the advancement of the 3D printing field and the advantages of the overall approach as being a low-cost, easy-to-produce, and easy-to-deploy alternative to the current methods, 3D printed microfluidic systems for sweat collection and on-chip analysis will be a game changer in the future. Moreover, the role of computational studies can support game changer platforms by analyzing and predicting 3D printing materials.
Computational methods in designing materials for 3D printing and advancing performance of sensors
In the past decade, novel technologies have been developed and applied by researchers for biomarker detection. Reactions extensively happen between analytes in solution and receptors on a surface [73]. Microfluidic systems include continuous flow regimes in micron-sized channels that are developed for different biological and chemical applications [74]. Due to the limitation of experimental methods at the microscopic level, a combination of in silico designs with experimental studies has immense potential in developing biosensors [75]. This part of the review exhibits an overview of the applications of computation methods, particularly DFT to study and the design of 3D printed microfluidic biosensors. Furthermore, for more clear understanding of these methods, Table 1 briefly presents some comparisons and properties of computational and simulation methods.
Briefly, Yin et al. designed a portable fluorescence sensor using high-transparency resin and 3D printing technology for sensitive detection of multiple pathogenic bacteria. Using fluorescent probes (tetraphenylethylene (TPE) derivatives) caused bacteria to emit fluorescence with different colors. By injecting fluorescent bacteria into a microfluidic device, the number of fluorescent bacteria was counted. Photosensitive resin was used as the synthetic material for 3D printing. Different fluorescence of three TPE derivatives were proven using DFT computations. Figure 4 presents the results of these computations on TPE and TPE derivatives including TPE-COOH, TPE-B-COOH, and TPE-X-COOH [95].
DFT-calculated HOMO and LUMO values of TPE derivative. Reprinted with permission [95]. Copyright 2023, Elsevier
Litti et al. presented a 3D printed microfluidic chip combined with Janus magnetic/plasmonic nanostars Fe3O4/Au (JMNSs) as surface-enhanced Raman scattering (SERS) colloidal substrates. In this study, several small-molecule analytes were selected, such as a dye with antifungal and antibacterial activity, the standard pH-sensitive molecule p-mercaptobenzoic acid (MBA), a clinically relevant anticancer drug, and herbicide flumioxazin. DFT computations were performed to achieve the Raman spectrum of flumioxazin [96]. Santangelo et al. fabricated a disposable 3D printed lab-on-chip based on epitaxial graphene for heavy metals detection. The 3D printed microfluidic chip interface led to the interaction between the sample solution and the graphene sensing surface. DFT computations were employed for the evaluation of conductivity changes and the sensing mechanism [97]. In spite of the significance of computation and in silico studies and their advantages in decreasing of manufacturing costs, and in the validation of experimental results through accurate computations, however, there are too limited studies about the application of computation in the study and design of 3D printed microfluidic biosensors. In the following, some DFT computations and MD simulations that were carried out to study of 3D printing material are mentioned as a suggestion for more use of these methods in the design of 3D printed microfluidic biosensors. Sun et al. prepared PDMS elastomer with good mechanical strength and self-healing function. They found that their powder-based 3D printing of PDMS elastomer as one the most investigated materials contains hindered pyrazole urea dynamic bonds. Using DFT computations and experiments of small molecule models, they studied the dynamic chemical mechanism of these hindered pyrazole urea bonds [98]. Using a heating-accelerated in situ gelation mechanism and direct ink writing (DIW) technique, Sun et al. reported a strategy to achieve rapid 3D printing of thermosets (silicone Sylgard 184). Heating-accelerated gelation of the thermosetting ink is the underlying principle of their technique. Using DFT computations, they showed that heating reduces the energy barrier for crosslinking and led to the rapid DIW of various thermosetting materials with heterogeneous structures, complex geometries, and multifunctionality [99].
Otieno et al. used both experimental studies and ab initio structural computations to characterize the structural, optical, mechanical, and electronic properties of PLA and ABS. The obtained PLA and ABS via lab-scale filament extrusion showed tensile strengths of 39.07 and 16.12 MPa. In addition, using DFT computations, they achieved the value of 1.899 and 2.539 eV for the band structure of PLA and ABS, and this result indicates the poor conductivity of both materials [100]. Due to properties such as high loading capability and excellent biocompatibility, silica-based mesoporous systems attract attention in drug delivery applications. Pore-size limitation is however one of the challenges in using of these materials to host large molecules such as proteins and enzymes. Considering bone remodeling, Banche-Niclot et al. developed large-pore mesoporous silicas (LPMSs) and used horseradish peroxidase (HRP) as a model enzyme to evaluate the ability of the LPMSs in the adsorption and the release of large biomolecules. By coating of LPMSs with poly (ethylene glycol) (PEG), they indicated the release of biomolecules in response to a pH decrease. Using DFT computations, they determined the pore size distribution of LMPSs and introduced the PEG-coated large mesoporous silica for protein delivery and its application in collagen-based formulation for 3D printing [101]. Tran et al. introduced modified graphene oxide as a gas barrier using polymer epoxy on 3D printing ABS substrates. Using experimental methods such as Raman, atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS), as well as DFT and MD simulations, their graphene epoxy-coated 3D printing substrates exhibited excellent barrier properties for O2. They estimated energy barriers of O2 in graphene nanopores using DFT computations and studied O2 penetration in nanopores using MD simulations [102]. Finding new materials for bone tissue repair is one of the important tasks of modern medicine. For the development of materials, 3D printing offers great opportunities. Stepanova et al. reported the characterization and biological development of 3D scaffolds based on poly (ε-caprolactone) (PCL) loaded with ciprofloxacin or dexamethasone. Preparation of drug-loaded PCL scaffolds by direct 3D printing from a polymer/drug blend was the novelty of their work. They applied DFT computations to calculate the pore characteristics of the 3D printed matrices. The results of computations validated their experimental studies about the effects of drugs on porous characterizes and specific surface area of 3D printed scaffolds [103].
Challenges and future perspectives
As previously expounded, the utilization of 3D printed microfluidic systems constitutes a paradigm replete with advantages for the meticulous analysis of sweat constituents. Nonetheless, akin to any technological modality, these methodologies are not devoid of inherent challenges. Surmounting these impediments through inventive stratagems possesses the latent capacity to augment the holistic efficacy of the system across multifarious dimensions. A conspicuous challenge resides in the circumscribed array of materials amenable to 3D printing endeavors, with a pronounced emphasis on applications within the purview of biological studies. The imperative necessitates a discerning focus on the exploration and refinement of materials characterized by elevated biocompatibility, particularly in the sphere of implantable devices [104]. Furthermore, the salience of compatibility between these materials and assorted apparatus, such as printed circuit boards (PCBs), cannot be overstated [105]. The integration of hybrid ceramic polymers emerges as a sanguine avenue for augmenting material attributes, especially in contexts mandating optical transparency [106]. Similarly, advancements in mechanical and chemical resistance can be achieved by optimizing curing agent ratios for polydimethylsiloxane (PDMS) molds [107]. The pursuit of new materials and refining existing ones is pivotal for overcoming these challenges and expanding the capabilities of 3D printed microfluidic systems in diverse applications.
The judicious execution of surface modification stands out as a preeminent concern for optimizing the efficacy and dependability of a microfluidic device [108]. Concurrently, the selection of a 3D printing material necessitates meticulous consideration of its compatibility with these surface modifications. A pivotal facet pertaining to both material composition and spatial configuration within the microfluidic framework is the efficacy in fluid collection from perspired exudates. Given the intrinsic variability in the rate of perspiration among individuals and across diverse anatomical regions in the body, the capacity to adeptly harvest a substantial volume of sweat at a designated site augments the sensor’s efficiency by amplifying the quantity of targeted analytes. A pervasive challenge being inherent in 3D printing resides in the dearth of standardized protocols and validated correlations between disparate printing devices and materials. Mitigating this challenge requires a strategic approach, wherein validation endeavors are propelled by ML algorithms that continually scrutinize and adapt to variations in printing techniques, printer parameters, materials employed, and subsequent analysis outcomes post-applications. While advancements in materials science and biosensor technologies remain imperative, complementary impetus can be garnered through robust support for computational studies, thereby fostering a symbiotic relationship between empirical innovation and computational acumen.
Conclusions
The integration of 3D printing with microfluidics points out a significant advancement in various sensor systems, particularly in the context of sweat analysis. These integrated systems present notable advantages, including expedited manufacturing, cost-effectiveness, and the capacity for continuous monitoring of biomarkers. As materials science progresses and optimization processes refine, 3D printed microfluidic platforms are poised to expand their applicability further. Sweat analysis, on the other hand, emerges as a preferable alternative to blood sample analysis, particularly for populations such as newborns and elderly patients, for whom can be discomforting. The analysis of sweat as a bodily fluid finds applications in disease diagnosis, as well as routine activities like daily walking and sports practice. These integrated systems hold a pivotal position in biomedical research and, notably, disease detection and analysis methods at the home or the point-of-care settings, offering promising prospects for human health. Crucially, for both patients and caregivers, the desirability of wearable systems that enable continuous and autonomous analysis, coupled with the ability to share these analyses via NFC, Wi-Fi, or Bluetooth with devices such as phones or computers, is paramount. Moreover, due to the economic difficulties experienced worldwide, it is essential that everyone has access to such low-cost systems. In addition to accessibility, the easy interpretability of these systems holds great promise for rapid disease detection.
Data availability
Not applicable.
References
Yildiz UH, Inci F, Wang S et al (2015) Recent advances in micro/nanotechnologies for global control of hepatitis B infection. Biotechnol Adv 33:178–190
Oliver NS, Toumazou C, Cass AEG, Johnston DG (2009) Glucose sensors: a review of current and emerging technology. Diabet Med 26:197–210. https://doi.org/10.1111/j.1464-5491.2008.02642.x
Erdem Ö, Derin E, Sagdic K et al (2021) Smart materials-integrated sensor technologies for COVID-19 diagnosis. Emergent Mater 4:169–185. https://doi.org/10.1007/s42247-020-00150-w
Ece E, Hacıosmanoğlu N, Inci F (2023) Microfluidics as a ray of hope for microplastic pollution. Biosensors 13:332. https://doi.org/10.3390/bios13030332
Mena-Bravo A, Luque de Castro MD (2014) Sweat: a sample with limited present applications and promising future in metabolomics. J Pharm Biomed Anal 90:139–147. https://doi.org/10.1016/j.jpba.2013.10.048
Jadoon S, Karim S, Akram MR et al (2015) Recent developments in sweat analysis and its applications. Int J Anal Chem 2015:1–7. https://doi.org/10.1155/2015/164974
Biscay J, Findlay E, Dennany L (2021) Electrochemical monitoring of alcohol in sweat. Talanta 224:121815. https://doi.org/10.1016/j.talanta.2020.121815
Osilla E, Marsidi J, Shumway K, Sharma S (2023) Physiology, temperature regulation. StatPearls
Jagannath B, Lin K, Pali M et al (2021) Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng Transl Med 6:e10220
Zafar H, Channa A, Jeoti V, Stojanović GM (2022) Comprehensive review on wearable sweat-glucose sensors for continuous glucose monitoring. Sensors 22:638
Surapongchai J, Saengsirisuwan V, Rollo I et al (2021) Hydration status, fluid intake, sweat rate, and sweat sodium concentration in recreational tropical native runners. Nutrients 13:1374
Liu Y, Shen H, Yang X, Kang S, Cai L, Tian T, Su R, Yang C, Zhu Z et al (2023) Recent progress in microfluidic biosensors with different driving forces. TrAC Trends Anal Chem 158:116894. https://doi.org/10.1016/j.trac.2022.116894
Akceoglu GA, Saylan Y, Inci F (2021) A snapshot of microfluidics in point‐of‐care diagnostics: multifaceted integrity with materials and sensors. Adv Mater Technol 6. https://doi.org/10.1002/admt.202100049
Niculescu A-G, Chircov C, Bîrcă AC, Grumezescu AM (2021) Fabrication and applications of microfluidic devices: a review. Int J Mol Sci 22:2011
Erdem Ö, Eş I, Saylan Y et al (2023) In situ synthesis and dynamic simulation of molecularly imprinted polymeric nanoparticles on a micro-reactor system. Nat Commun 14:4840
Duong LH, Chen P et al (2019) Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics 13(2):024108. https://doi.org/10.1063/1.5092529
Quan H, Zhang T, Xu H et al (2020) Photo-curing 3D printing technique and its challenges. Bioact Mater 5:110–115
Bunea A-I, del Castillo Iniesta N, Droumpali A et al (2021) Micro 3D printing by two-photon polymerization: configurations and parameters for the nanoscribe system. In: Micro. MDPI, pp 164–180. https://doi.org/10.3390/micro1020013
Kristiawan RB, Imaduddin F, Ariawan D et al (2021) A review on the fused deposition modeling (FDM) 3D printing: filament processing, materials, and printing parameters. Open Eng 11:639–649
Kadry H, Wadnap S, Xu C, Ahsan F (2019) Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur J Pharm Sci 135:60–67
Patpatiya P, Chaudhary K, Shastri A, Sharma S (2022) A review on PolyJet 3D printing of polymers and multi-material structures. Proc Inst Mech Eng Part C J Mech Eng Sci 236:7899–7926
Wang K, Li S, Rao Y et al (2019) Flexure behaviors of ABS-based composites containing carbon and Kevlar fibers by material extrusion 3D printing. Polymers (Basel) 11:1878
Inci F (2022) Benchmarking a microfluidic-based filtration for isolating biological particles. Langmuir 38:1897–1909. https://doi.org/10.1021/acs.langmuir.1c03119
Tümer EH, Erbil HY (2021) Extrusion-based 3D printing applications of PLA composites: a review. Coatings 11:390
Guo J, Zeng Y, Li P, Chen J (2019) Fine lattice structural titanium dioxide ceramic produced by DLP 3D printing. Ceram Int 45:23007–23012
Jiang R, Monteil L, Kimes K et al (2021) Influence of powder type and binder saturation on binder jet 3D–printed and sintered Inconel 625 samples. Int J Adv Manuf Technol 116:3827–3838
Sanei SHR, Popescu D (2020) 3D-printed carbon fiber reinforced polymer composites: a systematic review. J Compos Sci 4:98
Mathur K, Kabir SMF, Seyam A-FM (2022) Tensile properties of 3D printed continuous fiberglass reinforced cellular composites. J Text Inst 113:60–69
Guo C, Zhang M, Devahastin S (2021) Color/aroma changes of 3D-printed buckwheat dough with yellow flesh peach as triggered by microwave heating of gelatin-gum Arabic complex coacervates. Food Hydrocoll 112:106358
Pessoa S, Guimarães AS, Lucas SS, Simões N (2021) 3D printing in the construction industry-a systematic review of the thermal performance in buildings. Renew Sustain Energy Rev 141:110794
Kamyshny A, Magdassi S (2019) Conductive nanomaterials for 2D and 3D printed flexible electronics. Chem Soc Rev 48:1712–1740
Hamp SM, Logan RD, Shaw JA (2021) Optical transmittance of 3D printing materials. Appl Opt 60:6573–6578
Dagdag O, Hsissou R, El Harfi A et al (2020) Fabrication of polymer based epoxy resin as effective anti-corrosive coating for steel: computational modeling reinforced experimental studies. Surfaces and Interfaces 18:100454
Sagdic K, Es I, Sitti M, Inci F et al (2022) Smart materials: rational design in biosystems via artificial intelligence. Trends Biotechnol 987–1003. https://doi.org/10.1016/j.tibtech.2022.01.005
Yetisen AK, Moreddu R, Seifi S et al (2019) Dermal tattoo biosensors for colorimetric metabolite detection. Angew Chemie 131:10616–10623
Dang W, Manjakkal L, Navaraj WT et al (2018) Stretchable wireless system for sweat pH monitoring. Biosens Bioelectron 107:192–202
Zhou W, He Q, Ye H et al (2021) Recent advances in flexible sweat glucose biosensors. J Phys D Appl Phys 54:423001
Inci F, Saylan Y, Kojouri AM et al (2020) A disposable microfluidic-integrated hand-held plasmonic platform for protein detection. Appl Mater Today 18:100478. https://doi.org/10.1016/j.apmt.2019.100478
Nagamine K, Nomura A, Ichimura Y et al (2020) Printed organic transistor-based biosensors for non-invasive sweat analysis. Anal Sci 36:291–302
Gao F, Liu C, Zhang L et al (2023) Wearable and flexible electrochemical sensors for sweat analysis: a review. Microsystems Nanoeng 9:1
Bariya M, Nyein HYY, Javey A (2018) Wearable sweat sensors Nat Electron 1:160–171. https://doi.org/10.1038/s41928-018-0043-y
Xu J, Fang Y, Chen J (2021) Wearable biosensors for non-invasive sweat diagnostics. Biosensors 11:245. https://doi.org/10.3390/bios11080245
Sonner Z, Wilder E, Heikenfeld J, et al (2015) The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 9. https://doi.org/10.1063/1.4921039
Nyein HYY, Bariya M, Tran B et al (2021) A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat Commun 12:1823
Viswanathan S, Narayanan TN, Aran K et al (2015) Graphene–protein field effect biosensors: glucose sensing. Mater Today 18:513–522
Wang L, Pumera M (2021) Recent advances of 3D printing in analytical chemistry: focus on microfluidic, separation, and extraction devices. TrAC Trends Anal Chem 135:116151
Jin Y, Xiong P, Xu T, Wang J (2022) Time-efficient fabrication method for 3D-printed microfluidic devices. Sci Rep 12:1233
Prabhakar P, Sen RK, Dwivedi N et al (2021) 3D-printed microfluidics and potential biomedical applications. Front Nanotechnol 3:609355
Poskus MD, Wang T, Deng Y et al (2022) Digital Light Processing 3D printing for biological applications of polydimethylsiloxane-based microfluidics. BioRxiv. https://doi.org/10.1101/2022.09.28.509779
Ali MA, Hu C, Yttri EA, Panat R (2022) Recent advances in 3D printing of biomedical sensing devices. Adv Funct Mater 32:2107671
Razavi Bazaz S, Rouhi O, Raoufi MA et al (2020) 3D printing of inertial microfluidic devices. Sci Rep 10:5929
Aladese AD, Jeong H-H (2021) Recent developments in 3D printing of droplet-based microfluidics. BioChip J 15:313–333
Ho CMB, Ng SH, Li KHH, Yoon Y-J (2015) 3D printed microfluidics for biological applications. Lab Chip 15:3627–3637
Bhattacharjee N, Urrios A, Kang S, Folch A (2016) The upcoming 3D-printing revolution in microfluidics. Lab Chip 16:1720–1742
Kabandana GM, Zhang T, Chen C et al (2022) Emerging 3D printing technologies and methodologies for microfluidic development. Anal Methods 2885–2906. https://doi.org/10.1039/D2AY00798C
Macdonald NP, Cabot JM, Smejkal P et al (2017) Comparing microfluidic performance of three-dimensional (3D) printing platforms. Anal Chem 89:3858–3866
Karakurt I, Lin L (2020) 3D printing technologies: techniques, materials, and post-processing. Curr Opin Chem Eng 28:134–143
Su R, Wen J, Su Q (2020) 3D printed self-supporting elastomeric structures for multifunctional microfluidics. Sci Adv 6:eabc9846
Vijayan S, Parthiban P, Hashimoto M (2021) Evaluation of lateral and vertical dimensions of micromolds fabricated by a PolyJet™ printer. Micromachines 12:302. https://doi.org/10.3390/mi12030302
Amin R, Knowlton S, Hart A et al (2016) 3D-printed microfluidic devices. Biofabrication 8:22001
Romanov V, Samuel R, Chaharlang M et al (2018) FDM 3D printing of high-pressure, heat-resistant, transparent microfluidic devices. Anal Chem 90:10450–10456
Ahmed R, Ozen MO, Karaaslan MG, et al (2020) Tunable fano‐resonant metasurfaces on a disposable plastic‐template for multimodal and multiplex biosensing. Adv Mater 32. https://doi.org/10.1002/adma.201907160
Li F, Smejkal P, Macdonald NP et al (2017) One-step fabrication of a microfluidic device with an integrated membrane and embedded reagents by multimaterial 3D printing. Anal Chem 89:4701–4707
Mehta V, Rath SN (2021) 3D printed microfluidic devices: a review focused on four fundamental manufacturing approaches and implications on the field of healthcare. Bio-Design Manuf 4:311–343
Zhang J, Xiao P (2018) 3D printing of photopolymers. Polym Chem 9:1530–1540
Kitamori H, Sumida I, Tsujimoto T et al (2019) Evaluation of mouthpiece fixation devices for head and neck radiotherapy patients fabricated in PolyJet photopolymer by a 3D printer. Phys Medica 58:90–98
Wu S, Serbin J, Gu M (2006) Two-photon polymerisation for three-dimensional micro-fabrication. J Photochem Photobiol A Chem 181:1–11. https://doi.org/10.1016/j.jphotochem.2006.03.004
Wei L, Fang G, Kuang Z et al (2022) 3D-printed low-cost fabrication and facile integration of flexible epidermal microfluidics platform. Sensors Actuators B Chem 353:131085. https://doi.org/10.1016/j.snb.2021.131085
Kim T, Yi Q, Hoang E, Esfandyarpour R (2021) A 3D printed wearable bioelectronic patch for multi-sensing and in situ sweat electrolyte monitoring. Adv Mater Technol 6:1–11. https://doi.org/10.1002/admt.202001021
Yang DS, Wu Y, Kanatzidis EE et al (2023) 3D-printed epidermal sweat microfluidic systems with integrated microcuvettes for precise spectroscopic and fluorometric biochemical assays. Mater Horizons 10:4992–5003. https://doi.org/10.1039/D3MH00876B
Roda A, Guardigli M, Calabria D et al (2014) A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 139:6494–6501. https://doi.org/10.1039/c4an01612b
Nah JS, Barman SC, Zahed MA et al (2021) A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sensors Actuators, B Chem 329:129206. https://doi.org/10.1016/j.snb.2020.129206
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biotechnol 26:417–426. https://doi.org/10.1038/nbt1388
Luka G, Ahmadi A, Najjaran H et al (2015) Microfluidics integrated biosensors: a leading technology towards lab-on-a-chip and sensing applications. Sensors (Switzerland) 15:30011–30031. https://doi.org/10.3390/s151229783
Aslan Y, Atabay M, Chowdhury HK, et al (2023) Aptamer-based point-of-care devices: emerging technologies and integration of computational methods. Biosensors 13. https://doi.org/10.3390/bios13050569
Assadi MHN, Hanaor DAH (2013) Theoretical study on copper’s energetics and magnetism in TiO2 polymorphs. J Appl Phys 113. https://doi.org/10.1063/1.4811539
Hanaor DAH, Assadi MHN, Li S et al (2012) Ab initio study of phase stability in doped TiO2. Comput Mech 50:185–194. https://doi.org/10.1007/s00466-012-0728-4
Segall MD, Lindan PJD, Probert MJ et al (2002) First-principles simulation: ideas, illustrations and the CASTEP code. J Phys Condens Matter 14:2717–2744. https://doi.org/10.1088/0953-8984/14/11/301
Music D, Geyer RW, Schneider JM (2016) Recent progress and new directions in density functional theory based design of hard coatings. Surf Coatings Technol 286:178–190. https://doi.org/10.1016/j.surfcoat.2015.12.021
Orio M, Pantazis DA, Neese F (2009) Density functional theory. Photosynth Res 102:443–453
Sundararaman R, Letchworth-Weaver K, Schwarz KA et al (2017) JDFTx: software for joint density-functional theory. SoftwareX 6:278–284. https://doi.org/10.1016/j.softx.2017.10.006
Baez JC, May JP (2010) Towards higher categories. Springer, New York, New York, NY
Koehl P, Levitt M (1999) A brighter future for protein structure prediction. Nat Struct Biol 6:108–111
Hatmal MM, Jaber S, Taha MO (2016) Combining molecular dynamics simulation and ligand-receptor contacts analysis as a new approach for pharmacophore modeling: beta-secretase 1 and check point kinase 1 as case studies. J Comput Aided Mol Des 30:1149–1163. https://doi.org/10.1007/s10822-016-9984-2
Suzuki J, Tadashi N, Moore MJ (2017) Modeling, methodologies and tools for molecular and nano-scale communications: modeling, methodologies and tools. Springer Cham. https://doi.org/10.1007/978-3-319-50688-3
Goldman BB, Wipke WT (2000) QSD quadratic shape descriptors. 2. Molecular docking using quadratic shape descriptors (QSDock). Proteins Struct Funct Genet 38:79–94. https://doi.org/10.1002/(SICI)1097-0134(20000101)38:1%3c79::AID-PROT9%3e3.0.CO;2-U
Meng EC, Shoichet BK, Kuntz ID (1992) Automated docking with grid-based energy evaluation. J Comput Chem 13:505–524. https://doi.org/10.1002/jcc.540130412
Feig M, Onufriev A, Lee MS et al (2004) Performance comparison of generalized born and Poisson methods in the calculation of electrostatic solvation energies for protein structures. J Comput Chem 25:265–284. https://doi.org/10.1002/jcc.10378
Torres PHM, Sodero ACR, Jofily P, Silva-Jr FP (2019) Key topics in molecular docking for drug design. Int J Mol Sci 20:4574. https://doi.org/10.3390/ijms20184574
Basharat Z, Bibi M, Yasmin A et al (2020) Implications of molecular docking assay for bioremediation. IGI Global 22. https://doi.org/10.4018/978-1-7998-1204-3.ch078
Suresh PS, Kumar A, Kumar R, Singh VP (2008) An in silco approach to bioremediation: laccase as a case study. J Mol Graph Model 26:845–849. https://doi.org/10.1016/j.jmgm.2007.05.005
Fan J, Fu A, Zhang L (2019) Progress in molecular docking. Quant Biol 7:83–89. https://doi.org/10.1007/s40484-019-0172-y
Yan Y, Zhang D, Zhou P et al (2017) HDOCK: a web server for protein–protein and protein–DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res 45:W365–W373. https://doi.org/10.1093/nar/gkx407
Grosdidier A, Zoete V, Michielin O (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39:W270–W277. https://doi.org/10.1093/nar/gkr366
Yin P, Wang J, Li T et al (2023) A smartphone-based fluorescent sensor for rapid detection of multiple pathogenic bacteria. Biosens Bioelectron 242:115744. https://doi.org/10.1016/j.bios.2023.115744
Litti L, Trivini S, Ferraro D, Reguera J (2021) 3D printed microfluidic device for magnetic trapping and SERS quantitative evaluation of environmental and biomedical analytes. ACS Appl Mater Interfaces 13:34752–34761. https://doi.org/10.1021/acsami.1c09771
Santangelo MF, Shtepliuk I, Filippini D et al (2019) Real-time sensing of lead with epitaxial graphene-integrated microfluidic devices. Sensors Actuators, B Chem 288:425–431. https://doi.org/10.1016/j.snb.2019.03.021
Sun S, Fei G, Wang X et al (2021) Covalent adaptable networks of polydimethylsiloxane elastomer for selective laser sintering 3D printing. Chem Eng J 412:128675. https://doi.org/10.1016/j.cej.2021.128675
Sun Y, Wang L, Ni Y, et al (2023) 3D printing of thermosets with diverse rheological and functional applicabilities. Nat Commun 14. https://doi.org/10.1038/s41467-023-35929-y
Otieno DB, Bosire GO, Onyari JM, Mwabora JM (2022) Comparative analysis of 3D-printed polylactic acid and acrylonitrile butadiene styrene: experimental and Materials-Studio-based theoretical studies. J Polym Res 29:1–14. https://doi.org/10.1007/s10965-022-03075-6
Banche-Niclot F, Montalbano G, Fiorilli S, Vitale-Brovarone C (2021) PEG-coated large mesoporous silicas as smart platform for protein delivery and their use in a collagen-based formulation for 3D printing. Int J Mol Sci 22:1–18. https://doi.org/10.3390/ijms22041718
Tran NA, Jang S, Joo SW (2019) Graphene epoxy spray on 3D-printed acrylonitrile butadiene styrene substrates as O2 gas barrier. Appl Surf Sci 497:143745. https://doi.org/10.1016/j.apsusc.2019.143745
Stepanova M, Averianov I, Gofman I et al (2023) Drug loaded 3D-printed poly (ε-caprolactone) scaffolds for local antibacterial or anti-inflammatory treatment in bone regeneration. Polymers (Basel) 15:3957
Yilmaz EG, Ece E, Erdem Ö et al (2023) A sustainable solution to skin diseases: ecofriendly transdermal patches. Pharmaceutics 15:579. https://doi.org/10.3390/pharmaceutics15020579
Erdem Ö, Derin E, Zeibi Shirejini S et al (2022) Carbon-based nanomaterials and sensing tools for wearable health monitoring devices. Adv Mater Technol 7:2100572
Sikanen T, Aura S, Heikkila L et al (2010) Hybrid ceramic polymers: new, nonbiofouling, and optically transparent materials for microfluidics. Anal Chem 82:3874–3882
Kim M, Moon B-U, Hidrovo CH (2013) Enhancement of the thermo-mechanical properties of PDMS molds for the hot embossing of PMMA microfluidic devices. J Micromechanics Microengineering 23:95024
Tokel O, Inci F, Demirci U (2014) Advances in plasmonic technologies for point of care applications. Chem Rev 114:5728–5752
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Dr. Fatih Inci, Kadriye Ölmez, and Nedim Hacıosmanoğlu gratefully acknowledge the support of TÜBİTAK 2232 − International Fellowship for Outstanding Researchers (project no: 118C254). This publication has been produced benefiting from the 2232 International Fellowship for Outstanding Researchers Program of TÜBİTAK (project no: 118C254). However, the entire responsibility for the article belongs to the owner of the article. The financial support received from TÜBİTAK does not mean that the content of the publication is approved in a scientific sense by TÜBİTAK. Dr. Fatih Inci also acknowledges the support of GEBIP Award from Turkish Academy of Sciences (TÜBA). This work was also supported by BAGEP Award of the Science Academy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ece, E., Ölmez, K., Hacıosmanoğlu, N. et al. Advancing 3D printed microfluidics with computational methods for sweat analysis. Microchim Acta 191, 162 (2024). https://doi.org/10.1007/s00604-024-06231-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06231-5